An increasing number of patients are taking antiplatelet or anticoagulation medications, or both. These drugs are prescribed to those who are at high risk for or who have had a thromboembolic event (e.g., blood clot). They include patients who have experienced a pulmonary embolism, deep-vein thrombosis or have nonvalvular atrial fibrillation, a cardiac arrhythmia that predisposes them to clot formation. Anticoagulants include the vitamin K antagonist warfarin and the newer direct oral agents, including the thrombin inhibitor dabigatran and factor Xa inhibitors.1-5 Antiplatelet agents include clopidogrel, ticlopidine, prasugrel, ticagrelor and aspirin.6
Despite their safety, the use of anticoagulation agents when minimally invasive dental procedures are required remains a source of discomfort for dental practitioners and a common reason for referral to specialists. Adverse effects that dissuade dentists from treating patients taking antithrombotics are prolonged bleeding and bruising. However, without anticoagulant/antiplatelet medications, these patients are at higher risk for blood-clot development, which could result in thromboembolism, stroke or myocardial infarction. Therefore, the risks of stopping or reducing these medications must be weighed against the potential consequences of prolonged bleeding.7-12 A thorough understanding of why these medications are prescribed in outpatient medical practice, as well as their implications for dental care, is essential for providing optimal oral health care.
Blood Coagulation Cascade
To appreciate the mechanism of action of specific anticoagulant drugs, health care professionals must understand the process of coagulation. This is especially important with the introduction of new direct-acting oral anticoagulants (DOACs), which are specific tissue-clotting factors.
Hemostasis is a complex physiological process that limits blood loss at a site of tissue injury, without disrupting normal blood flow elsewhere.13,14 The coagulation cascade, with the end point of hemostasis, is composed of an intrinsic and an extrinsic pathway (Figure 1). Each pathway involves specific factors, but they converge to convert factor X into its activated form, factor Xa. Factor Xa is required for the conversion of prothrombin into thrombin, which, in turn, is required for the conversion of fibrinogen into fibrin. This ultimately leads to the formation of a fibrin clot. As blood coagulation is a cascade, inhibition of any factor will inhibit subsequent events.
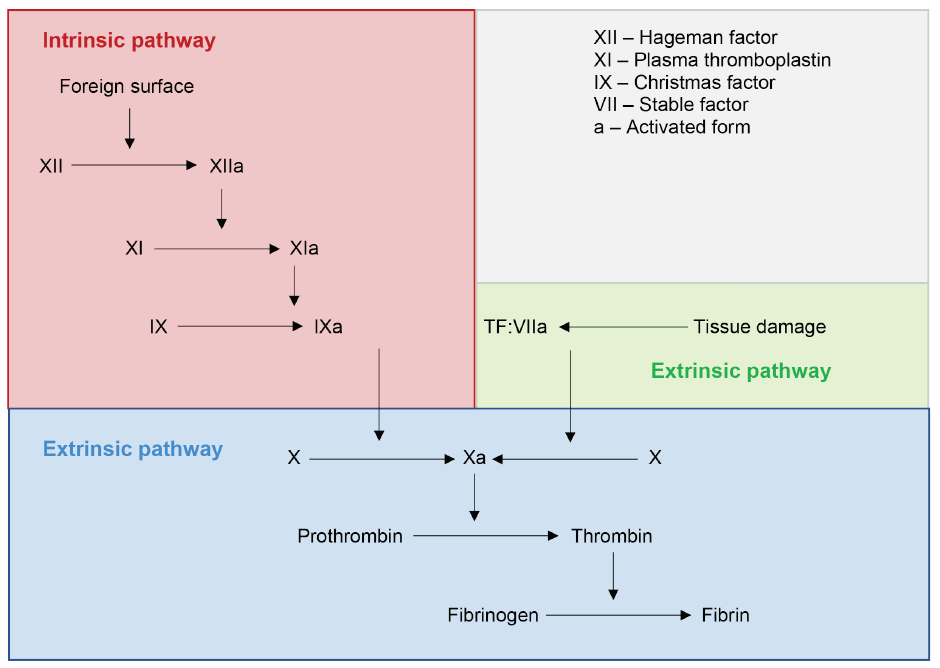
Figure 1: Classic coagulation cascade.
Source: Adapted from Pallister and Watson MS.15
Vitamin K Antagonists
Vitamin K antagonists are a group of drugs that inhibit blood clotting by reducing the active form of vitamin K, through inhibition of the enzyme vitamin K epoxide reductase. Vitamin K is required for the γ carboxylation of vitamin K-dependent coagulation factors II, VII, IX, X and proteins C and S, which activate them.14 When warfarin is first administered, a few days are required to achieve therapeutic effects. This is because it inhibits the formation of new clotting factors but does not affect previously formed factors. To monitor the effectiveness of a vitamin K antagonist or to assess the blood’s ability to clot, a prothrombin time test is indicated. For the common vitamin K antagonist, warfarin, the result is expressed as the international normalized ratio (INR). INR is a measure of warfarin’s therapeutic index and is used to determine how quickly blood clots, with higher numbers indicating a longer time to achieve hemostasis. In healthy patients, an INR below 1.1 is considered normal. An INR of 2.0–3.0 is generally an effective therapeutic range for patients taking medication for atrial fibrillation to prevent thromboembolic events.16 In some circumstances, such as when a patient has a mechanical heart valve, an even higher INR may be indicated.
It is important for the informed practitioner to know a patient’s INR if they are taking a vitamin K antagonist, as this will help guide clinical decisions. Ideally, the patient should obtain their INR value on the day of their dental procedure. A 2015 systematic review of management of dental extractions in patients receiving warfarin determined that patients whose INR was within therapeutic range (i.e., 3.0 or less) could continue their regular warfarin regimen before the procedure.17 An earlier systematic review found that continuing the regular dose of warfarin did not seem to increase risk of bleeding compared with discontinuing or modifying the warfarin dose for patients undergoing minor dental procedures.18 Reversal of the effect of vitamin K antagonists can be achieved via administration of vitamin K, fresh frozen plasma or prothrombin complex concentrate, but this should only be done after consultation with a physician.
Direct-Acting Oral Anticoagulants
The clinical use of DOACs offers benefits because of their predictable, targeted mechanism of action toward a single coagulation factor (Table 1). DOACs are classified as either direct thrombin inhibitors or factor Xa inhibitors; they exhibit rapid onset of action, swift elimination, a wide therapeutic margin and they have a shorter half-life and fewer drug interactions.19,20 Both are metabolized primarily by the hepatic and renal systems; therefore, those suffering from renal impairment or elderly people will experience a longer half-life compared with younger people. Because DOACs are generally prescribed as fixed-dose regimens, they also do not require routine monitoring. These advantages are the reason why a greater number of physicians are prescribing DOACs rather than vitamin K antagonists for patients in need.21
Even though most adverse events involving excessive anticoagulant activity can be controlled by drug discontinuation because of the short half-life, dentists must be prepared to manage severe hemorrhage during extensive surgical procedures, such as multiple extractions (> 3 teeth in 1 session), subgingival scaling, crown lengthening, surgical extractions, periodontal surgery and open-flap surgical procedures. In cases where severe bleeding is expected, dentists should be prepared to administer DOAC reversal agents, such as idarucizumab for dabigatran and andexanet alfa for apixaban and rivaroxaban.
Thrombin (Factor IIa) Inhibitors: Dabigatran — Direct thrombin inhibitors reversibly bind to the active site on thrombin to inactivate both its free and bound forms. These inhibitors, the most common being dabigatran, are currently approved by the United States’ Food and Drug Administration (FDA) for prevention of cerebrovascular events and venous thromboembolism in non-valvular atrial fibrillation.22 Dabigatran is a prodrug (dabigatran etexilate) administered orally twice a day in doses of either 110 mg or 150 mg. It reaches peak plasma concentration in 1.5 h and has a half-life of 12–17 h, relying on the renal and hepatic systems for metabolism.
Factor Xa Inhibitors: Rivaroxaban, Apixaban, Edoxaban — Factor Xa inhibitors block the interaction of thrombin by binding to the active site on factor Xa in both its soluble and bound forms in the prothrombinase complex.19 They are commonly prescribed for patients requiring treatment of deep vein thrombosis, prevention of venous thromboembolism, prevention of stroke and patients with other systemic embolic disorders.23 These medications are generally administered orally once a day, or sometimes twice if taken immediately after surgery, and show a rapid onset of 1.5–3 h depending on the specific drug. The best laboratory test to monitor the anticoagulant effect of factor Xa inhibitors is an anti-factor Xa chromogenic assay. Although routine monitoring is often not required, physicians may recommend it for obese patients, underweight patients or those with renal failure.24-26
Vitamin K Antagonists vs. Direct-Acting Oral Anticoagulants
The primary difference between these two groups of drugs is their mechanism of action (Figure 1 and Table 1). DOACs are increasing in popularity because they require no routine monitoring and are considered safer and more predictable because of their direct effect on the coagulation cascade (targeting 1 specific factor). The shortened half-life of DOACs may also be seen as an advantage when it comes to temporary withdrawal of anticoagulant therapy before major surgery, although it can be a disadvantage and dangerous if patients do not take their medication.27
One study found that, with DOACs, the decrease in the number of INR tests required on a routine basis could save countries with an established public health care system millions of dollars each year.28 In contrast, with warfarin, INR testing must be done frequently as warfarin’s effects vary greatly depending on systemic levels of vitamin K (e.g., antibiotics reduce the level of vitamin K-producing gut bacteria). Although DOACs offer many advantages over vitamin K antagonists, it is important to note that a “gold standard” anticoagulant does not exist.
To answer the question “which is safer?” a 2016 study29 of patients undergoing DOAC therapy compared with those undergoing regular anticoagulant therapy (vitamin K antagonists) found no significant difference in number of post-extraction bleeding events (defined as hemorrhage lasting longer than 20 minutes) between the 2 groups. Furthermore, 91.7% of the bleeding events were mild and could be controlled by applying pressure to the bleeding site with gauze. The remaining 8.3% required re-approximating the wound margins, applying a fibrin sealant and then re-suturing. The results of this study suggest that dental extractions and less-invasive procedures may be safely performed using local hemostatic measures, if necessary, without modifying or interrupting ongoing oral anticoagulant therapy.
Antiplatelet Drugs
Antiplatelet drugs are also used to prevent thrombus formation, but they affect a different biologic pathway than the previously discussed drugs. For thrombogenesis to occur, a platelet plug is formed as a primary means to inhibit hemostasis. However, platelets aggregate only when activated by binding to specific factors. Many antiplatelet agents work by inhibiting the formation of important molecules in this process or by blocking receptors. Clopidogrel, ticlopidine, prasugrel and ticagrelor inhibit ADP-induced expression of GpIIb/IIIa, which is required for platelets to bind to fibrinogen and to other platelets, by irreversibly binding to and blocking the P2Y12 receptor. Aspirin, another commonly used antiplatelet drug, works by inhibiting cyclooxygenase 2 (COX-2) and, thereby, the production of TXA2, which plays a variety of roles in platelet aggregation. Aspirin irreversibly inhibits COX in platelets, preventing them from aggregating for their lifetime (8–9 days). It is most commonly taken in the low-dose form as a preventive agent, mainly for myocardial infarction and ischemic stroke.30 Current recommendations do not suggest discontinuing the use of daily aspirin before routine dental extractions, including multiple routine extractions.31 Recommendations also state that the thromboembolic risk of discontinuing aspirin and clopidogrel likely outweighs potential bleeding complications associated with surgery.8
In rare circumstances, where a patient is believed to be at high hemorrhagic risk, the dentist should consider requesting a consultation with the patient’s physician to discuss temporarily discontinuing the drug therapy. Such situations may pertain to patients with qualitative or quantitative platelet disorders, coagulopathies, chronic kidney or liver failure and alcoholics.32-38 If, after the medical consultation, the patient is deemed to be at too high a risk to discontinue antiplatelet therapy, the patient should be referred to receive treatment in a hospital setting. A hospital is better equipped to handle severe bleeding events and complications associated with the patient’s complex medical condition(s).
Drug Interactions with Commonly Prescribed Drugs in Dentistry
Warfarin — The vitamin K antagonist warfarin is biotransformed by cytochrome P450 (CYP) enzymes, a common pathway for a variety of drugs. Drugs that inhibit CYP enzymes effectively increase the risk of major bleeding events by indirectly decreasing warfarin metabolism. These drugs include azole antifungals and macrolide antibiotics (see Table 1 for specific drugs). The literature is less clear regarding the relation between acetaminophen and warfarin, with some reports suggesting an increase in bleeding risk while others suggest no significant change in the patient’s INR.39 Monitoring may be indicated only in warfarin patients taking acetaminophen daily for time spans longer than 1 week. Clinicians should be aware that prescribing nonsteroidal anti-inflammatory drugs (NSAIDs) concurrently with warfarin can cause complications as it can increase the patient’s INR more than 15%, which can lead to gastrointestinal bleeding.40 In general, NSAIDs should be avoided in patients taking warfarin. However, in situations where patients require NSAIDs and cannot be managed using other therapies, selective COX-2 inhibitors are associated with fewer hospital admissions for gastrointestinal bleeding.41
Dabigatran — The thrombin (factor IIa) inhibitor, dabigatran, is a substrate for the efflux transporter, P-glycoprotein (P-gp). Drugs that also act on P-gp make the therapeutic effect of dabigatran unpredictable, which could put patients at higher risk of thromboembolic events. Drugs that induce P-gp, such as rifampin and dexamethasone, should not be administered at the same time as they have been observed to lower the average plasma concentration and peak serum concentrations of dabigatran. Drugs that inhibit P-gp, such as ketoconazole and clarithromycin, should also be avoided, if possible, because of their ability to significantly increase the average plasma concentration and peak serum concentration of dabigatran. In cases where there is a strong indication for the prescription of P-gp inhibitors, the physician should consider temporarily lowering the dose of dabigatran to reduce the risk of serious bleeding events. As for warfarin, dentists should consider additional monitoring of patients taking dabigatran and non-COX selective NSAIDs.
Rivaroxaban, Apixaban and Edoxaban — Like dabigatran, the remaining DOACs are also substrates for the efflux transporter P-gp, but they differ in that they are metabolized by multiple CYP enzymes (primarily CYP3A4) and CYP-independent mechanisms. Drugs that strongly inhibit CYP enzymes or the P-gp transporter, such as azole antifungals, should be avoided in patients taking rivaroxaban, apixaban or edoxaban, as they will markedly increase the plasma concentrations of these DOACs and, therefore, increase the risk of hemorrhage either intra- or post-operatively. Drugs that mainly inhibit only 1 elimination pathway, such as clarithromycin or erythromycin, have been shown to slightly increase the average plasma concentration and peak serum concentration; however, this effect is not clinically relevant and, therefore, concomitant use is considered safe.42 With regard to concomitant use of NSAIDs and these DOACs, research demonstrated that bleeding time was significantly increased; however, this did not have any clinically significant effect.43
How to Manage Dental Perioperative Bleeding
Although many dental procedures can be performed while patients are taking antithrombotic medications, precautions must be taken to minimize perioperative bleeding. It is important for clinicians to understand the invasiveness of the procedure they are performing, the bleeding risk it poses and the patient’s current medical history. Low-risk dental procedures, such as routine scaling, restorations that involve limited soft tissue manipulation, extractions that are not surgically complex and involve < 3 teeth, soft tissue biopsies, endodontic procedures, simple implant placement, fixed and removable dentures, crowns and bridges do not require an alteration in anticoagulant medications. If warfarin is used, INR values should be within the therapeutic range whenever possible.11,44-46 Procedures associated with high bleeding risk include periodontal surgery, surgical extractions, multiple extractions (> 3 teeth) and osteoplasty. These procedures may require an alteration in anticoagulant medications and should be performed only after consultation with the patient’s physician. Patients who are taking oral anticoagulants and have co-existing medical problems that affect hemostasis, such as liver disease, renal disease or thrombocytopenia, may have an even greater risk of bleeding. Consequently, an updated and thorough medical history is of utmost importance.
Most bleeding can be controlled by the use of both mechanical and pharmacologic local hemostatic measures. For most patients who take antithrombotics, local pressure with gauze compression is adequate to achieve hemostasis.46 If further measures are indicated, suturing, electrocautery, local anesthetic containing epinephrine, styptics, oxidized cellulose, absorbable sponges and bone wax are recommended, as each has hemostatic effects.46,48 Cellulose and absorbable sponges act by providing a matrix for platelet adhesion and activation. Epinephrine in local anesthetic acts as a chemical tourniquet by causing local vasoconstriction, helping to minimize blood loss. Electric cauterization may be indicated if a larger blood vessel becomes lacerated and fails to form a thrombus. Bone wax is used as a tamponade when the source of the bleeding is from the bone; the amount used should be as minimal as possible, as the wax is not resorbable. Bone wax is a foreign body and may elicit an inflammatory response (foreign body giant cell reaction), impaired osteogenesis and/or increased susceptibility to infection.49
For extraction, tooth sockets should be gently packed with an absorbable hemostatic dressing (e.g., oxidized cellulose, collagen sponge, absorbable gelatin sponge, or platelet-rich fibrin), then carefully sutured. Following closure, pressure should be applied to the socket(s) using a gauze pad and having the patient bite down on it for as long as 1 h to achieve hemostasis. Further bleeding control can be attained, if necessary, by placing a tea bag in the socket and the patient biting on it for 30 minutes; the tannic acid in regular tea serves as a local vasoconstrictor.50
If bleeding concerns remain, oral topical antifibrinolytics, such as e aminocaproic acid or tranexamic acid, can be used as an adjunct.43 A regimen of 10 mL of a 4.8% tranexamic acid solution 4 times a day for 7 days has been shown to reduce bleeding postoperatively.51 However, a typical prescription for an antifibrinolytic could represent a significant cost to the patient, and this must be considered before prescribing.
Patients should be given clear instructions on the management of the clot in the postoperative period including: avoid vigorous rinsing, spitting, sucking or chewing on the affected side and, if bleeding continues or restarts, apply pressure using a folded piece of gauze for about 20 minutes. Finally, clinicians should counsel patients postoperatively about the signs and symptoms of excessive bleeding that should prompt them to seek further care at their dental office. Figure 2 is a visual representation of the steps in the management of postoperative bleeding.

Figure 2: Treatment management for patients undergoing antithrombotic therapy
Conclusion
There is a general consensus that, in most cases, treatment regimens with older anticoagulants (e.g., warfarin), antiplatelet agents (e.g., ticagrelor, ticlopidine, prasugrel, clopidogrel and/or aspirin) and new DOACs (e.g., dabigatran, rivaroxaban, apixaban and edoxaban) should not be altered before routine dental procedures with a low bleeding risk. The risks of ceasing or reducing these medication regimens (e.g., myocardial infarction, thromboembolism and stroke) far outweigh the consequences of prolonged hemorrhage, which can be controlled with local hemostatic measures. This approach is based on risk reduction by aiming to simplify patient management and minimize adverse clinical outcomes.
Because new DOACs have only recently been approved by the FDA, more research is required before a definitive periprocedural treatment strategy for high-risk bleeding can be developed. Any potential alterations to the patient’s medication regimen before dental treatment should be planned in consultation with the patient’s physician. Over time, oral health care providers can expect to encounter many more patients being treated with DOACs compared with older anticoagulants, because of the advantages they offer, such as increased predictability and shortened half-life. It is, therefore, critical for clinicians to be familiar with the mechanism of thrombus formation; the reasons why patients are prescribed antiplatelet and/or anticoagulant medications; and pre-operative, intra-operative and post-operative management. For more concise information regarding this subject, please see the American Dental Association52 and Thrombosis Canada.53
|
|
Warfarin (Coumadin) |
Dabigatran (Pradaxa) |
Rivaroxaban (Xarelto) |
Apixaban (Eliquis) |
Edoxaban (Savaysa) |
|---|---|---|---|---|---|
|
Note: INR = international normalized ratio, NSAIDs = nonsteroidal anti-inflammatory drugs. |
|||||
| Dose(s) | 1 mg daily 4 mg daily |
110 mg twice daily 150 mg twice daily |
20 mg daily | 5 mg twice daily | 15 mg daily 30 mg daily 60 mg daily |
| Mechanism of action | Vitamin K antagonist | Thrombin (factor IIa) inhibitor | Factor Xa inhibitor | Factor Xa inhibitor | Factor Xa inhibitor |
| Bioavailability | 100% | 6–7.2% | 80% | 50% | 62% |
| Peak plasma concentration time | 48–72 h | 1.5 h | 3 h | 3–4 h | 1.5 h |
| Elimination half-life | 36–42 h | 12–17 h | 7–11 h | 8–15 h | 9–10 h |
| Metabolism | Hepatic | 80% renal 20% hepatic |
Renal | 55% hepatic 25% renal |
62.2% hepatic 35.4% renal |
| Monitoring | Yes | No | No | No | No |
| Recommended coagulation test | INR | Thrombin time Activated thromboplastin time |
Prothrombin time Anti-factor Xa chromogenic assay |
||
| Common drug interactions | |||||
| Potentiators | Ciprofloxacin Erythromycin Azithromycin Fluconazole Metronidazole Amiodarone NSAIDs |
Amiodarone Verapamil Digoxin Ketoconazole Clarithromycin Aspirin Dexamethasone |
Itraconazole Fluconazole Ketoconazole Clarithromycin Other anticoagulants |
||
| Inhibitors | Barbiturates Carbamazepine Rifampin Nafcillin |
St. John’s Wort Rifampin Antacids |
St. John’s Wort Phenytoin Carbamazepine Phenobarbital |
||
THE AUTHORS
Correspondence to: Dr. Aviv Ouanounou, Department of Clinical Sciences, Pharmacology & Preventive Dentistry, Faculty of Dentistry, University of Toronto, 124 Edward St, Room 513, Toronto ON M5G 1G6. Email: aviv.ouanounou@dentistry.utoronto.ca
The authors have no declared financial interests in any company manufacturing the types of products mentioned in this article.
This article has been peer reviewed.
References
- Prescribing information: PRADAXA (dabigatran etexilate) capsules, for oral use. Ridgefield, Ct.: Boehringer Ingelheim; 2020. Accessed 2020 Nov. 9. Available from: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf
- Prescribing information: ELIQUIS (apixaban) tablets, for oral use. Princeton, NJ: Bristol-Myers Squibb; 2019. Accessed 2020 Nov. 9. Available from: https://packageinserts.bms.com/pi/pi_eliquis.pdf
- Prescribing information: SAVAYSA (edoxaban) tablets, for oral use. Tokyo: Dalichi Sankyo; 2015. Accessed 2020 Nov. 9. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf
- Prescribing information: XARELTO (rivaroxaban) tablets, for oral use. Leverkusen, Germany: Janssen Pharmaceuticals; 2019. Accessed 2020 Nov. 9. Available from: http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/XARELTO-pi.pdf
- Which oral anticoagulant for atrial fibrillation? JAMA. 2016;315(19):2117-8.
- Gurbel PA, Myat A, Kubica J, Tantry US. State of the art: oral antiplatelet therapy. JRSM Cardiovasc. Dis. 2016;5:2048004016652514.
- Napeñas JJ, Hong CH, Brennan MT, Furney SL, Fox PC, Lockhart PB. The frequency of bleeding complications after invasive dental treatment in patients receiving single and dual antiplatelet therapy. J Am Dent Assoc. 2009;140(6):690-5.
- Grines CL, Bonow RO, Casey Jr DE, Gardner TJ, Lockhart PB, Moliterno DJ, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Coll Cardiol. 2007;49(6):734-9.
- Grines CL, Bonow RO, Casey Jr DE, Gardner TJ, Lockhart PB, Moliterno DJ, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115(6):813-8.
- Thean D, Alberghini M. Anticoagulant therapy and its impact on dental patients: a review. Aust Dent J. 2016;61(2):149-56.
- Napeñas JJ, Oost FC, DeGroot A, Loven B, Hong CH, Brennan MT, et al. Review of postoperative bleeding risk in dental patients on antiplatelet therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(4);491-9.
- Jeske AH, Suchko GD. Lack of a scientific basis for routine discontinuation of oral anticoagulation therapy before dental treatment. J Am Dent Assoc. 2003;134(11):1492-7.
- Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363-70.
- Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet. 2015;386(9990):281-91.
- Pallister CJ, Watson MS. Overview of haemostasis. In: Haematology. 2nd ed. Bloxham, UK: Scion Publishing; 2010. p. 336-47.
- Reynolds MW, Fahrbach K, Hauch O, Wygant G, Estok R, Cella C, et al. Warfarin anticoagulation and outcomes in patients with atrial fibrillation: a systematic review and metaanalysis. Chest. 2004;126(6):1938-45.
- Weltman NJ, Al-Attar Y, Cheung J, Duncan DP, Katchky A, Azarpazhooh A, et al. Management of dental extractions in patients taking warfarin as anticoagulant treatment: a systematic review. J Can Dent Assoc. 2015;81:f20.
- Nematullah A, Alabousi A, Blanas N, Douketis JD, Sutherland SE. Dental surgery for patients on anticoagulant therapy with warfarin: a systematic review and meta-analysis. J Can Dent Assoc. 2009;75(1):41.
- Fakhri HR, Janket SJ, Jackson EA, Baird AE, Dinnocenzo R, Meurman JH. Tutorial in oral antithrombotic therapy: biology and dental implications. Med Oral Patol Oral Cir Bucal. 2013;18(3):e461-71.
- Fitzgerald JL, Howes LG. Drug interactions of direct-acting oral anticoagulants. Drug Saf. 2016;39(9):841-5.
- Gómez‐Outes A, Suárez‐Gea ML, Lecumberri R, Terleira‐Fernández AI, Vargas‐Castrillón E. Direct‐acting oral anticoagulants: pharmacology, indications, management, and future perspectives. Eur J Haematol. 2015;95(5):389-404.
- Blommel ML, Blommel AL. Dabigatran etexilate: a novel oral direct thrombin inhibitor. Am J Health Syst Pharm. 2011;68(16):1506-19.
- Firriolo FJ, Hupp WS. Beyond warfarin: the new generation of oral anticoagulants and their implications for the management of dental patients. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(4):431-41.
- Levy JH, Key NS, Azran MS. Novel oral anticoagulants: implications in the perioperative setting. Anesthesiology. 2010;113(3):726-45.
- Zikria JC, Ansell J. Oral anticoagulation with factor Xa and thrombin inhibitors: on the threshold of change. Curr Opin Hematol. 2009;16(5):347-56.
- Singh M, Adigopula S, Patel P, Kiran K, Khosla S. Recent advances in oral anticoagulation for atrial fibrillation. Ther Adv Cardiovasc Dis. 2010;4(6):395-407.
- Dentali F, Riva N, Crowther M, Turpie AG, Lip GY, Ageno W. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126(20):2381-91.
- Aalbers J. Anti-thrombotic trials in atrial fibrillation, the RELY study. Cardiovasc J Afr. 2010;21(5):299.
- Mauprivez C, Khonsari RH, Razouk O, Goudot P, Lesclous P, Descroix V. Management of dental extraction in patients undergoing anticoagulant oral direct treatment: a pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122(5):e146-55.
- Patrono C, García Rodríguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353(22):2373-83.
- Brennan MT, Wynn RL, Miller CS. Aspirin and bleeding in dentistry: an update and recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(3):316-23.
- Barbui T, Buelli M, Cortelazzo S, Viero P, De Gaetano G. Aspirin and risk of bleeding in patients with thrombocythemia. Am J Med. 1987;83(2):265-8.
- Pullar T, Capell HA. Interaction between oral anti-coagulant drugs and non-steroidal anti-inflammatory agents: a review. Scott Med J. 1983;28(1):42-7.
- Escolar G, Cases A, Bastida E, Garrido M, Lopez J, Revert, L., et al. Uremic platelets have a functional defect affecting the interaction of von Willebrand factor with glycoprotein IIb-IIIa. Blood. 1990;76(7):1336-40.
- Thomason JM, Seymour RA, Murphy P, Brigham KM, Jones P. Aspirin‐induced post‐gingivectomy haemorrhage: a timely reminder. J Clin Periodontol. 1997;24(2):136-8.
- Mannucci PM, Vicente V, Vianello L, Cattaneo M, Alberca I, Coccato MP, et al. Controlled trial of desmopressin in liver cirrhosis and other conditions associated with a prolonged bleeding time. Blood. 1986;67(4):1148-53.
- Deykin D, Janson P, McMahon L. Ethanol potentiation of aspirin-induced prolongation of the bleeding time. N Engl J Med. 1982;306(14):852-4.
- Rosove MH, Harwig SS. Confirmation that ethanol potentiates aspirin-induced prolongation of the bleeding time. Thromb Res. 1983;31(3):525-7.
- Hughes GJ, Patel PN, Saxena N. Effect of acetaminophen on international normalized ratio in patients receiving warfarin therapy. Pharmacotherapy. 2011;31(6):591-7.
- Choi KH, Kim AJ, Son IJ, Kim KH, Kim KB, Ahn H, et al. Risk factors of drug interaction between warfarin and nonsteroidal anti-inflammatory drugs in practical setting. J Korean Med Sci. 2010;25(3):337-41.
- Cheetham TC, Levy G, Niu F, Bixler F. Gastrointestinal safety of nonsteroidal antiinflammatory drugs and selective cyclooxygenase-2 inhibitors in patients on warfarin. Ann Pharmacother. 2009;43(11):1765-73.
- Gnoth MJ, Buetehorn U, Muenster U, Schwarz T, Sandmann S. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. 2011;338(1):372-80.
- Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Rivaroxaban (BAY 59‐7939) — an oral, direct factor Xa inhibitor — has no clinically relevant interaction with naproxen. Br J Clin Pharmacol. 2007;63(4):469-76.
- Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl.):e326S-50S.
- Spyropoulos AC, Al‐Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-85.
- Perry DJ, Noakes TJ, Helliwell PS. Guidelines for the management of patients on oral anticoagulants requiring dental surgery. Br. Dent J. 2007;203(7):389-93.
- Bajkin BV, Selaković SD, Mirković SM, Šarčev IN, Tadić AJ, Milekić BR. Comparison of efficacy of local hemostatic modalities in anticoagulated patients undergoing tooth extractions. Vojnosanit Pregl. 2014;71(12):1097-101.
- Lim W, Wang M, Crowther M, Douketis J. The management of anticoagulated patients requiring dental extraction: a cross‐sectional survey of oral and maxillofacial surgeons and hematologists. J Thromb Haemost. 2007;5(10):2157-9.
- Schonauer C, Tessitore E, Barbagallo G, Albanese V, Moraci A. The use of local agents: bone wax, gelatin, collagen, oxidized cellulose. Eur Spine J. 2004;13(suppl. 1):S89-96.
- Hupp JR, Ellis E, Tucker MR. Contemporary Oral and Maxillofacial Surgery. 6th ed. St. Louis, Mo.: Mosby; 2013.
- Carter G, Goss A, Lloyd J, Tocchetti R. Tranexamic acid mouthwash versus autologous fibrin glue in patients taking warfarin undergoing dental extractions: a randomized prospective clinical study. J Oral Maxillofac Surg. 2003;61(12):1432-5.
- Oral anticoagulant and antiplatelet medications and dental procedures. American Dental Association; 2020. Accessed 2020 Nov 17. Available from: https://www.ada.org/en/member-center/oral-health-topics/anticoagulant-antiplatelet-medications-and-dental-procedures
- Thrombosiscanada.ca. Whitby, Ont.: Thrombosis Canada; 2020. Accessed 2020 Nov 9. Available from: https://thrombosiscanada.ca/