The success of non-surgical periodontal treatment depends on the removal of deposits from crown and root surfaces using mechanical debridement. To achieve this effectively, the periodontal instruments used must have high-quality cutting edges.1 However, edges become dull with use and the efficiency of the instrument is lost after a number of strokes.2-4 In fact, the cutting edge may become slightly rounded after as few as 15 strokes and, after 45 strokes, a very rounded edge is created.2,5 A rounded edge makes it difficult for the blade of the instrument to engage the deposit on the tooth surface and results in a greater chance of burnishing a deposit rather than removing it. Therefore, it is necessary to sharpen instruments at the first sign of dullness to maintain efficiency and ensure optimal treatment.6,7
Difficult cases and some types of procedures may require an instrument to be sharpened several times during a single treatment session using a sterile stone. Several authors have recommended that periodontal instruments be frequently sharpened or honed using sharpening stones during a debridement session to maintain the cutting edges in proper working condition.8,9 The maintenance of a sharp cutting edge during treatment provides numerous benefits, including increased tactile sensitivity for the operator, increased patient comfort and increased control of the instrument. Thus, reduced instrument slippage (i.e., an instrument sliding in a direction the operator did not intend and potentially causing damage) leads to improved safety for both the patient and operator.7,10,11 The ability to sharpen instruments at chair side ensures that these benefits will be realized.
The use of sharpening stones during patient care has been questioned by some professional organizations because of possible hazards of occupational exposure and lack of evidence that sharpening stones can be properly sterilized. Although no evidence in the literature supports these concerns, protocols have been prepared and enhanced by the same organizations.12 Exposures to risk during patient care in dentistry are relatively common.13-17 Needles and burs are the most common instruments associated with risk, while periodontal instruments are involved in about 10% of incidents.16 Incidents often relate to patient movement or improper handling or cleaning of instruments.14,18 We did not find any data that specifically deal with percutaneous injuries during instrument sharpening chair side nor any evidence that contaminated sharpening stones are not properly sterilized using the standard sterilization protocols recommended by the manufacturers.
We hypothesized that using the manufacturer’s protocol, sharpening stones can be sterilized. Furthermore, we approached the clinic director at the University of British Columbia (UBC) to find out the prevalence of incident reports among novice users of periodontal instruments.
Materials and Methods
Ceramic sharpening stones (medium grit; Hu-Friedy, Chicago, Ill. USA) were used for the study. Fifteen new stones were cleaned and sterilized according to the manufacturer’s protocol. Briefly, the stones were cleaned in a thermodisinfector and then placed in a EZ 10 dental sterilizer (Tuttnauer, Hauppauge, N.Y., USA) for 7 minutes at 134°C and 31 psi with a drying time of 31 minutes.
The stones were then divided into 3 groups of 5 stones. One group of stones (control) was sterilized as above but never used in patient care. A second group was used to sharpen dental curettes during a routine maintenance appointment in a periodontal office. Swabs were taken from the portion of the stone used for sharpening multiple instruments throughout the appointment. The remaining set of 5 stones was used as above but then cleaned and sterilized as described above. Swabs were taken from these instruments immediately after completion of the sterilization cycle. Stones were wiped with swabs designed for collection and transportation of bacterial specimens (BD BBL CultureSwab; Becton, Dickinson and Co., Franklin Lakes, N.J., USA).
Bacterial cultures were prepared as described previously.19 Briefly, the swabs were raised in 1 mL brain heart infusion culture medium (Sigma-Aldrich, Oakville, Ont.) and vortexed for a minimum of 1 minute to suspend the bacteria in the medium. The samples were diluted as necessary and seeded on 5% sheep’s blood agar plates (VWR International, Randor, Penn., USA). One agar plate per sample was incubated at 37°C for 3 days under aerobic conditions. A second plate was placed in an anaerobic pouch system (AnaeroGen; Oxoid, Basingstoke, UK) and incubated at 37°C for 7 days. Petri dishes were removed from the incubator after 3 or 7 days, and digital images (Rebel T1i; Canon, Tokyo, Japan) were taken for the purpose of counting bacterial colonies using the image processing software ImageJ 1.46r (National Institutes of Health, Rockville, Md., USA). Bacterial growth was recorded as either no growth (−) or growth (+). The Fisher exact test was then used to determine the efficiency of the sterilization protocol.
A set of sharpening stones treated as above was fixed in 2.5% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA, USA) in 0.1 M PIPES [piperazine-1,4-bis (2-ethanesulfonic acid)] buffer for 5 minutes and processed for scanning electron microscopy (SEM). A set of curettes used for periodontal maintenance debridement and then wiped with cotton gauze was also processed for SEM as above, to observe the presence of retained metal filings on the sharpened curette.
Results
New unused ceramic sharpening stones that were sterilized did not show any bacterial growth on either aerobic or anaerobic plating conditions (Table 1, Fig. 1). All new stones that were used once for chair-side sharpening during routine periodontal debridement showed bacterial growth both in aerobic and anaerobic culture conditions (Table 1, Fig. 1). As expected, the contamination level in terms of colony-forming units (CFUs) varied among the stones: 9–1600 CFUs per swab in aerobic and 24–2800 CFUs per swab in anaerobic conditions. When the used instruments were processed and sterilized using the recommended office protocol, all ceramic sharpening stones tested were found to be bacteria free (Table 1, Fig. 1). The efficiency of sterilization was then tested with Fisher’s exact test and found to be highly significant.
| Group | Stone number | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| New, never used (n = 5) | − | − | − | − | − |
| Used, not sterilized (n = 5) | + | + | + | + | + |
| Used, sterilized (n = 5) | − | − | − | − | − |
| New, Never Used |
Used, Not Sterilized |
Used, Sterilized |
|
|---|---|---|---|
| Aerobic |  |
||
| Anaerobic | |||
Figure 2: Scanning electron microscopy images showing (A) an unused sterilized ceramic sharpening stone, (B) a stone used for chair-side sharpening and (C) a stone that was used for chair-side sharpening and then cleaned and sterilized using normal office protocol. Debris is visible together with worn lattices of ceramic crystals in B (arrows). Debris is no longer present after sterilization; however, worn lattices remain on the stone surface (C, arrows).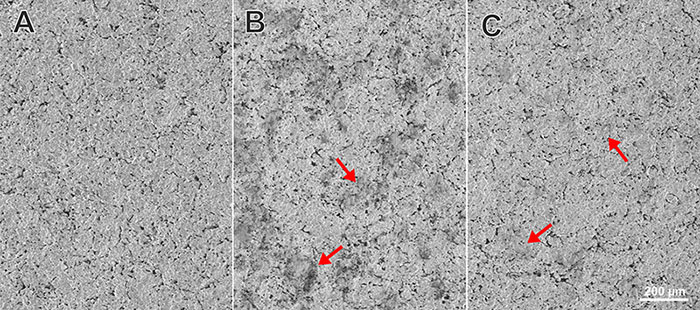
We then analyzed a set of ceramic stones that were new, used or used and sterilized. Under SEM, the unused and sterilized stones showed lattices of ceramic crystals (Fig. 2A). The stone that was used for sharpening at chair side showed worn crystal lattices and debris with particle size consistent with broken lattice particles, bacteria or metal filings (Fig. 2B). When a used stone was cleaned and sterilized, the debris was no longer present but the worn lattices were still retained on the surface as expected (Fig. 2C).
We also used SEM to examine the curettes that were used to reveal how much debris was present on the blade after instrumentation followed by sharpening and wiping with gauze. The used instrument had debris on the cutting edge and on the face of the blade (Fig. 3A). After sharpening and wiping, the instrument contained minimal debris and metal filing particles (Fig. 3B).
Figure 3: Representative scanning electron microscopy images of (A) a used curette showing debris on the cutting edge and face after sharpening (arrows); and (B) a curette showing minimal debris (arrows) after it was sharpened chair side and wiped with cotton gauze.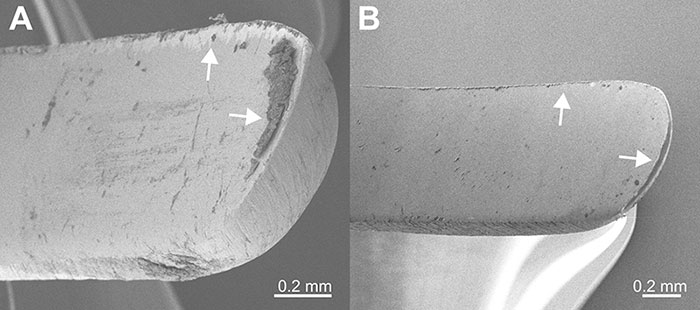
To determine the prevalence of occupational exposure during sharpening of periodontal instruments, we contacted the clinic director of the UBC dental clinics. No incident reports dealing with instrument sharpening, either in simulation or patient care, had been filed over the last year. These clinics include nearly 400 dental, hygiene and specialty students who can be considered novice users of dental instruments.
Discussion
The results of this quality-control study show that ceramic sharpening stones can be successfully sterilized using the recommended routine protocols. In addition, instruments that were sharpened and wiped with gauze during a chair-side session contained minimal metal debris.
These observations support the worldwide standards in periodontal instrumentation recommending sharpening/honing instruments during patient care.8,9 These standards have always emphasized the importance of chair-side maintenance of the cutting edge of periodontal instruments. Therefore, the organizations that have policies preventing chair-side sharpening/honing by their members should re-consider their regulations.
For example, in 2011, the College of Registered Dental Hygienists of Alberta published a new protocol to be incorporated into dental hygiene practices.20 These guidelines, which preclude chair-side sharpening, were updated again in 2016 and given more authority by a statement made by the executive environment officer of Alberta Health Services and the protocol was adopted at the University of Alberta Dental School.12,21 These protocols are enforced for hygienists in Alberta, putting them at risk of disciplinary action should they use the previously accepted standard of care of chair-side sharpening. Given the lack of references, research or incidents of clinical problems with chair-side sharpening, we recommend that traditional sterilization protocols as listed by the sharpening stone manufacturers be used and that chair-side sharpening be continued in clinical practice as well as taught in Canadian dental schools.
Using sharp instruments in patient care remains a hazard. Percutaneous injuries are relatively common and, although mainly caused by needles and burs, periodontal instruments are also involved in a small number of cases.15-17,22 Most injuries occur during intraoral patient care and less frequently in cleaning and handling the instruments after the procedure.22 A survey of UBC dental clinics revealed no such reports during the last year, suggesting that percutaneous exposures related to periodontal instruments arise from the instrumentation itself and not from sharpening. Therefore, recommendations that prevent chair-side sharpening/honing are not evidence informed and may lead to unnecessary duplication of instruments (when instrument becomes dull, a new instrument is used) and frequent sterilization cycles (i.e., sterilize instrument, then sharpen and then re-sterilize) and cost. Frequent sterilization itself may result in loss of sharpness of periodontal instruments, especially if they are made of carbon steel.23
Another potential hazard associated with chair-side sharpening might relate to the introduction of metal filings into the patient’s tissues. No data in the literature indicate the presence of metal particles in patient biopsies after treatment. Nevertheless, the sharpening standards should always include the common practice of wiping the sharpened/honed blade with gauze. A set of instrument tips was tested in this study and found to contain little debris after wiping with gauze, suggesting that the risk of particle transfer into patient tissues is relatively low if proper techniques are followed.
Conclusions
We conclude that ceramic sharpening stones can be sterilized using normal office protocols and, when proper protocols are followed, chair-side sharpening adds little risk beyond handling operatory or periodontal instruments during patient care. Filings from chair-side sharpened instruments do not appear to be retained when the instruments are wiped with gauze before being re-used in a patient.







