Over the last several decades, concerns about the possibility of adverse health effects from exposure to mercury in dental amalgams and the desire for improved esthetic dental restorations have led to a steady and rapid increase in the use of resin composite restorations.1-3 Since the late 1990s and early 2000s, there has been a strong trend in terms of decreased use of amalgams, countered by an increase in the use of resin composites.4 However, dental resin composites have an average replacement time of 5.7 years, mainly because of secondary caries and fracture of the restoration (Fig. 1).5 Therefore, despite the esthetic appeal and reduced preparation size of the restoration associated with resin composites,6 concerns over higher fracture rates, reduced longevity, prevalence of secondary caries and bacterial proliferation have emerged.2,7,8
Most research on resins has focused on physical processes that lead to degradation9; however, recently, attention to biochemical processes leading to degradation has increased.10-12 Resin composites contain ester links that are vulnerable to hydrolysis by esterase activity in the oral cavity.10,13-17 Biodegradation results in deterioration of the bulk structure of resin composites as well as the composite–tooth interface and the release of degradation products, such as methacrylic acid, triethylene glycol (TEG) and bishydroxy-propoxy-phenyl-propane (BisHPPP) (Fig. 2).10,12,17 These products have been shown to affect cariogenic bacterial growth and virulence.7,18-21 The compromised composite–tooth interface allows saliva and bacteria to infiltrate the spaces between the tooth and composite, exacerbating the effects of biodegradation, undermining the restoration and likely contributing to recurrent caries, hypersensitivity and pulpal inflammation.22-27
This review begins with an introduction to resin composites and adhesives, followed by an analysis of the biochemical stability of these materials when challenged with human salivary esterases. The effects of bacteria on the biochemical stability of resin composite restorations and the reciprocal effects of degradation by-products on the bacteria are then discussed.
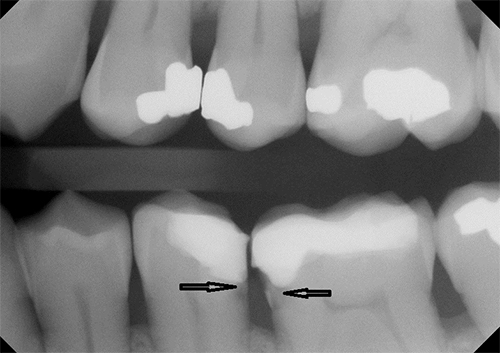 Figure 1: Radiographic image of secondary caries at the resin composite–tooth interface (arrows).
Figure 1: Radiographic image of secondary caries at the resin composite–tooth interface (arrows).
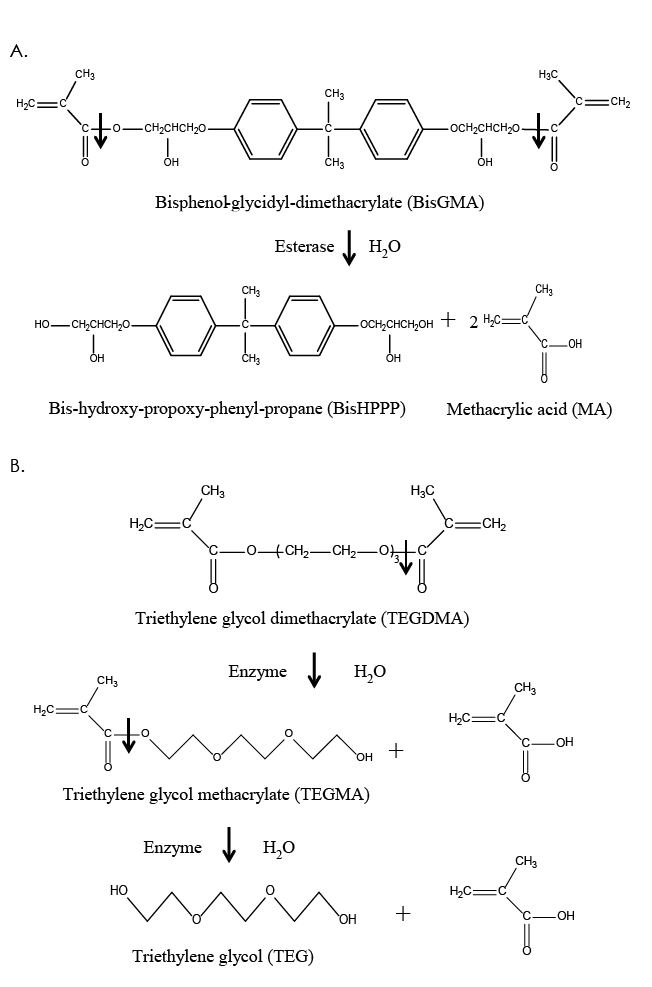 Figure 2: Structure and hydrolysis of the common dimethacrylate monomers, bisphenol-glycidyl-dimethacrylate (BisGMA) (a) and triethylene glycol dimethacrylate (TEGDMA) (b), by salivary and bacterial esterases, resulting in the production of the by-products, bishydroxy-propoxy-phenyl-propane (BisHPPP), triethylene glycol methacrylate (TEGMA), triethylene glycol (TEG) and methacrylic acid (MA).
Figure 2: Structure and hydrolysis of the common dimethacrylate monomers, bisphenol-glycidyl-dimethacrylate (BisGMA) (a) and triethylene glycol dimethacrylate (TEGDMA) (b), by salivary and bacterial esterases, resulting in the production of the by-products, bishydroxy-propoxy-phenyl-propane (BisHPPP), triethylene glycol methacrylate (TEGMA), triethylene glycol (TEG) and methacrylic acid (MA).
Resin Composites
The 4 constituents of dental resin composites are a polymeric matrix (usually methacrylate based); filler particles (usually glass, quartz or ceramic oxide, such as alumina or silica); coupling agents (used to improve bonding of the filler–polymer matrix); and a photoinitiator–inhibitor system.9,11,12,28
The Matrix
Dental resin monomers, which are the building blocks of the polymeric matrix, have been based primarily on the coupling of chemical moieties via ester links (Fig. 2). Some of the most popular resin monomers are bisphenol-glycidyl-dimethacrylate (BisGMA), triethylene glycol dimethacrylate (TEGDMA), urethane dimethacrylate (UDMA) and bisphenol A polyethylene glycol diether dimethacrylate (BisEMA) (Fig. 2).9,12,28
BisGMA is probably the most widely used monomer in both resin composites and adhesives. Its popularity is attributed to its relative non-volatility, low degree of polymerization shrinkage, rapid hardening under oral conditions and compatibility with current inorganic filler systems.9,12,28 A disadvantage of BisGMA is its high viscosity, which results from hydrogen bonds between hydroxyl groups in the alkyl chains and a rigid aromatic ring structure (Fig. 2). To facilitate handling and manipulation, various diluent monomers are used in conjunction with BisGMA, most commonly TEGDMA (Fig. 2), but other monomers, such as UDMA and BisEMA, are also used.9,12,28 The ratios and compositions of the monomers constituting resin composites vary depending on the application, location (anterior vs. posterior) of the restoration and the manufacturer’s preference.9,12,28
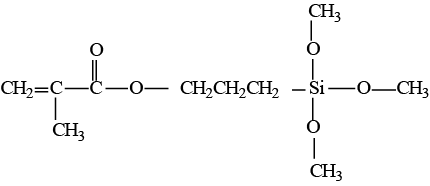 Figure 3: Structural formula of γ-methacryloxypropyltrimethoxy silane.
Figure 3: Structural formula of γ-methacryloxypropyltrimethoxy silane.
Filler Systems
Reinforcing fillers are major constituents of resin composites by weight and volume (about 50–80%). Fillers improve the physical properties of the composite, such as strength and modulus of elasticity, as well as reducing polymerization shrinkage, the coefficient of thermal expansion and water sorption.9,11,28,29
Coupling Agents
Coupling agents have been developed to increase the strength of the chemical link between the hydrophobic organic matrix and hydrophilic inorganic fillers. They have hydrophilic hydroxyl groups at one end and hydrophobic methacrylate groups at the other (Fig. 3). The most common coupling agents are organic silicon compounds called silanes.9,11,28
Polymerization Systems
Composites are converted from a viscous resin to a rigid solid through free radical polymerization of the methacrylate monomers. During polymerization, each molecule grows by the addition of a monomer to a terminal free radical reaction site. However, not all of the monomer’s double bonds will have reacted once polymerization has stopped. In fact, studies suggest that the degree of vinyl group conversion of the methacrylate materials ranges from 40% to 85%.11-13,30 The incomplete conversion is, in part, a result of a reduction in the diffusion rate of the propagating free radicals, the unreacted dimethacrylate molecules and the pendant methacrylate species as polymerization progresses.11,12,17 Thus, following the polymerization process, a significant amount of partly reacted and unreacted monomers remains in the polymeric matrix. Unreacted hydrophilic monomers may diffuse into saliva and interact with salivary proteins, bacteria and host tissue.7,10-14,16-21,26,31-34
Dental Resin Adhesives
Resin composite restorations require the application of adhesives to bond them efficiently to a tooth (specifically dentin). Dental resin adhesives are low-viscosity methacrylate-based liquids, which spread on the dentin surface and solidify to bond to the primed dentin on one side and to the resin composite substrate on the other.25,35 Dental adhesives and primers contain resin monomers that are similar to those found in the composite resin matrix by the inclusion of methacrylate functional groups, yet they contain hydrophilic moieties, such as hydroxyethyl methacrylate,25 to allow proper interactions with the tooth material. These adhesive monomers provide the covalent link between the adhesive and the composite, thus ensuring structural continuity and physical co-mechanical properties, such as strength. Currently, 2 types of adhesive systems are in use: total-etch (also known as etch-and-rinse) and self-etch systems.25
Total-Etch Adhesives
The first step in total-etch systems is the demineralization of the dentin surface to a depth of up to 20 μm by the application of an acid (usually 30–40% phosphoric acid), followed by a rinsing step and then the application of the primer and adhesive agents, which will infiltrate the exposed collagen and polymerize to form the resin–dentin interface or “hybrid layer.”25,36,37 The application of a primer followed by the adhesive agent successively in separate steps forms the basis for the 3-step etch-and-rinse technique. Simplified 2-step etch-and-rinse adhesives combine the primer and adhesive agent in 1 step.37
Selt-Etch Adhesives
A trend toward simpler techniques that are less practitioner sensitive and more time efficient has resulted in the manufacture of self-etch adhesives.25 These adhesives use non-rinse acidic monomers that simultaneously condition and prime dentin to form the hybrid layer. Self-etch adhesives may be 2-step or 1-step, depending on whether the hydrophobic adhesive resin is applied in a separate step or combined with the hydrophilic self-etch primer, respectively.38
Biodegradation of Dental Resin Composites and Adhesives
The degradation of dental resin composites and adhesives in the oral cavity is a result of many complex interactions, physical and biochemical. Most research has focused on the physical processes (food, chewing, etc.) that lead to degradation. These are classified as either material loss and uptake (sorption, extraction, dissolution and mineralization) or physical changes (softening, stress cracking, fatigue fracture, etc.).9 In contrast, biochemical processes have seldom been discussed until recently.
Biochemical processes that lead to degradation are thermolysis (decomposition caused by heat), oxidation (loss of electrons), solvolysis (decomposition caused by a solvent), photolysis (decomposition caused by light) and radiolysis (decomposition by ionizing radiation). Solvolysis, and more specifically hydrolysis when the solvent is water, is the most investigated and most relevant biochemical degradation process as it acts on the unprotected ester linkages in methacrylate-based resin monomers, polymers and coupling agents. By definition, hydrolysis is a chemical reaction during which water cleaves a molecule into two parts. One fragment of the parent molecule gains a hydrogen ion and the other a hydroxyl group from the water molecule (Fig. 2).9,10,12,17,31
The potential for enzymes to interact with resin composites and adhesives is significant. In the oral cavity, the enzymes most associated with the hydrolysis of resin composites and adhesives are esterases. Salivary esterases originate in the human gingival epithelium and salivary glands, through inflammatory responses and from microorganisms.9,10,16,17,32,33
Studies have shown the ability of human saliva to hydrolyze dental resin monomers, as well as the polymerized matrix.10,16,23,26,33,34,39 For example, Jaffer and colleagues17 showed that human saliva degrades the commercial composite resins Filtek Z250 (3M ESPE, St. Paul, MN, USA) and Spectrum TPH (L.D. Caulk, Milford, Del., USA), both of which contain BisGMA, TEGDMA and urethane-modified BisGMA12. In this study, standardized commercial photopolymerized composites were incubated with human saliva for up to 16 days (pH 7.0 and 37°C). Analysis of the incubation solutions revealed that human saliva catalyzed the biodegradation of both composites in a materials-dependent manner. Biodegradation products included methacrylic acid, TEGMA, BisHPPP and ethoxylated bisphenol A (E-BPA). The latter is a by-product of BisEMA biodegradation.12
The nature of degradation products was similar in both composite resins, but differences existed in the amounts of the products released. Both BisHPPP and methacrylic acid were produced in significantly greater amounts as a result of biodegradation of Z250 composite resin compared with TPH. However, more E-BPA was produced as a consequence of TPH degradation, although less than BisHPPP and methacrylic acid by-products. These differences can be explained by the variability of resin composite composition with respect to monomer types, monomer ratios, filler content and filler-to-resin ratios.11,12,40
Surface morphology of the cured composite was also found to contribute to the observed differences, as some configurations offer easier access to human salivary esterases and, therefore, more binding sites.11,12,40 Another factor may be the fact that urethane-modified BisGMA resin associated with TPH is more stable and resistant to hydrolysis and, thus, results in less release of degradation products.12 Furthermore, dental resin composites with higher silanated filler content have been shown to be less susceptible to the effects of degradation,11 unlike non-silanated filler particles, which are more susceptible, because of the lack of chemical bonding between the filler and the matrix.40 Therefore, the degradation product profile varies in terms of identity and amount depending on the composition of the resin composite or adhesive under investigation.
Biodegradation of the Resin–Dentin Interface
The resin–dentin interface is also susceptible to biodegradation.25,26,41-44 In a test of the effect of human saliva on total-etch adhesive, Shokati et al.26 incubated adhesive resin (Scotchbond Multi Purpose, 3M ESPE. St. Paul, MN, USA), resin composite (Z250, St. Paul, MN, USA) and resin composite–dentin mini short-rod specimens with human-saliva-derived esterases (HSDE) for up to 180 days (pH 7.0 and 37°C). The amount of a BisGMA-derived product, BisHPPP (Fig. 2), released into the incubation solutions was used as a marker of degradation of resin matrix and polymerized adhesive resin. Because BisGMA is a high molecular weight molecule with rigid phenyl rings and hydrogen bonding capacity, its diffusion through the resin matrix and out to the surface to interact with the enzymes is limited. Therefore, BisHPPP is a good indicator of resin matrix degradation.10-12,17,32
This work revealed that HSDE degraded both adhesive resin and resin composite to produce BisHPPP in comparable amounts. The strength of the resin composite–dentin bond was measured as the interfacial fracture toughness of mini short-rod specimens. Specimens incubated for 180 days in HSDE had the least resistance to fracture, while non-incubated specimens had the highest resistance. These results indicate that human salivary esterases compromise interfacial integrity by reducing the strength of the bond.23
In a more recent investigation, Serkies et al.,25 found similar effects on total-etch interfaces; however, self-etch specimens showed no reduction in fracture resistance even after 180-day incubation with simulated salivary esterase activities. The authors concluded that material composition and mode of application could affect the biochemical stability of the resin–dentin interface.
In another study, Kermanshahi et al.23 found an increase in the amount of BisHPPP and interfacial cariogenic bacterial microleakage following incubation of resin composite bonded to dentin using a total-etch system after incubation with salivary esterase-like activity for up to 90 days.
Using transmission electron microscopy, Jung et al.45 found increased nanoleakage in the resin–dentin interface after storage in an esterase solution compared with a phosphate buffer solution after 4 weeks. Using collagenase and acetylcholinesterase to simulate oral salivary enzymes, Chiaraputt et al.41 found that the microtensile bond strength of resin composite (Z350, 3M) bonded to human dentin using 4 different total-etch or self-etch adhesives was significantly lower when incubated in enzymes compared with incubation in water or not incubated. They also found that the self-etch adhesives exhibited “water-tree” patterns within the adhesive layer, probably because of the hydrophilicity of the adhesive,25,26 and that the total-etch adhesive exhibited nanoleakage within the hybrid layer and the adhesive, probably because of its incomplete penetration into the demineralized collagen network.25,26 Both phenomena compromised the interface, potentially affecting the restoration’s longevity.
These findings provide further evidence of biodegradation of the resin–dentin interface caused by salivary enzymes and show that the effect is materials dependent. These studies emphasize that alongside surface resin composite degradation, the resin–dentin interface is also degraded by human or simulated human salivary esterases.25,26,34,41-44
Biodegradation of Resin Composites and Adhesives by Bacteria and the Reciprocal Effect on the Bacteria
Although studies have investigated the impact of oral saliva on dental resin composites and adhesives, few have looked at the effect of bacteria on these substances. Such research has shown bacteria’s ability to penetrate microgaps caused by esterases and form colonies.
Kermanshahi et al.23 showed that exposing dentin–composite restorations bonded with total-etch adhesive to salivary esterase-like activity resulted in the formation of micro-gaps that were infiltrated and colonized by biofilms of the cariogenic bacteria Streptococcus mutans. Dental caries are believed to be the result of acid released by bacterial activity that leads to the demineralization of tooth structures. Streptococcus mutans has been found in dental plaque at the margins of composite fillings and is regarded as a major etiological agent responsible for dental caries.
Furthermore, Streptococcus mutans has been shown to exhibit esterase activity at levels capable of hydrolytic-mediated degradation of cured dental resin composites and adhesives.13 Bourbia et al.13 incubated a standard photopolymerized resin composite (Z250, 3M ESPE, St. Paul, MN, USA) and 2 adhesive resins — a total-etch (Scotchbond, 3M ESPE, St. Paul, MN, USA) and a self-etch (Easybond, 3M ESPE, St. Paul, MN, USA) resin — with the bacteria for up to 30 days. Following incubation, the medium contained the biodegradation by-product BisHPPP and the surface of the resins appeared rougher. The latter finding was in agreement with other investigations that showed an increase in surface degradation of dental resin composites incubated with bacteria (S. mutans, S. gordonii and S. sanguis).46-48
Overall, these results point toward the degradative effects of bacteria on dental resin composites and adhesives and highlight the potential for secondary caries and changes in esthetic properties seen clinically with the use of resin materials in dental restorations.
Bacteria-catalyzed biodegradation is further exacerbated by the effect of the by-products from dental resin composites and adhesives on the bacteria. Studies have revealed that by-products, such as TEG, increase bacterial growth and genetic expression of virulence.7,18 At levels found in vivo, TEG modulates the level of expression of glucosyltransferase B (gtfB), which is involved in biofilm formation, and yfiV, a putative transcription regulator.21
More recent studies have demonstrated a broader effect of TEG and BisHPPP on gene expression, as well as associated proteins related to the virulence of the cariogenic bacteria, Streptococcus mutans.19,20 These findings directly link biodegradation to bacterial proliferation in the oral cavity. This implies that resin composites, in their current form, are not only structurally vulnerable, but can also contribute to the progression of oral disease by increasing growth and virulence of the bacteria that grow over them and within the restoration–tooth margins.
On the other hand, other studies have found that BisGMA degradation products (BisHPPP and methacrylic acid) slightly inhibit growth of Streptococcus mutans,7,21 suggesting that different degradation products have different effects on oral bacteria and that the effect is strain-dependent. Therefore, to reach a convincing conclusion on the complete effect of degradation products on bacterial activity in the oral cavity, research must be conducted on the effect of cumulative degradation products on bacterial growth and virulence, also in multi-species cultures relevant to dental plaque.
Conclusion
Although dental resin composite and adhesive restoration materials have advantages, such as esthetic appeal, and no perceived danger of mercury poisoning, they seem to be at a disadvantage in terms of long-term stability, as they are affected by physical and biochemical challenges. Biochemical interactions may favour the proliferation of cariogenic bacteria and alter their virulence, thus potentially contributing to an increase in secondary caries and resin composite failure. Because the oral cavity is complex and various influences are acting in concurrence, it is evident that more research must be done to better understand the problems surrounding resin composites and adhesives to improve not only their physical properties, but also their biostability and interactions with oral biofilms.