The dentoalveolar complex is a common source of orofacial pain, and most cases of acute dental pain are successfully diagnosed and treated by general dentists. However, toothaches can recur or persist despite treatments judged to be adequate (Figure 1), and deciding whether any subsequent tooth-related treatments can relieve the patient’s symptoms is not an easy task. Such a presentation should remind the dentist that the pain might not be “dental” in nature even though the patient may point to a tooth.1 Lack of certainty about the absence of a dental cause of pain (for example, a possible cracked tooth or adjacent offending tooth) or the possibility of failed tooth-related treatment, despite a thorough history and diagnostic procedures, is often responsible for unnecessary or inappropriate dental treatment. This article presents a clinical reasoning process for improving the diagnostic and treatment approach for dentists managing patients with recurrent and persistent dental pain.
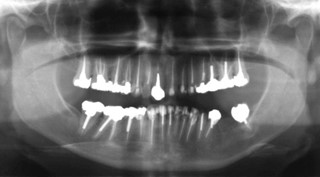
Figure 1: Panoramic image of the dentition of a 42-year-old female patient referred to an oral medicine specialist with multiple complaints, including pain in all her teeth.
Methods
Using PubMed, we conducted a selective literature review of relevant peer-reviewed publications written in English, beginning with the themes, “nonodontogenic tooth pain” and “persistent tooth pain,” as well as other key pain-related topics and factors influencing clinical decision-making. Both authors further identified and analyzed documents published by international organizations in the areas of pain and orofacial pain classification and definitions. The review also included key papers and textbook chapters selected from references cited by previously located articles to support premises presented in this paper.
Unravelling the Many Facets of Pain
Pain can be a complex and controversial issue for dentists and patients alike (Figure 2) and dentoalveolar pain is no exception (Figure 3). Pain is defined as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.”2 What is commonly overlooked is that not all pain is similar in terms of neurobiological processes.3
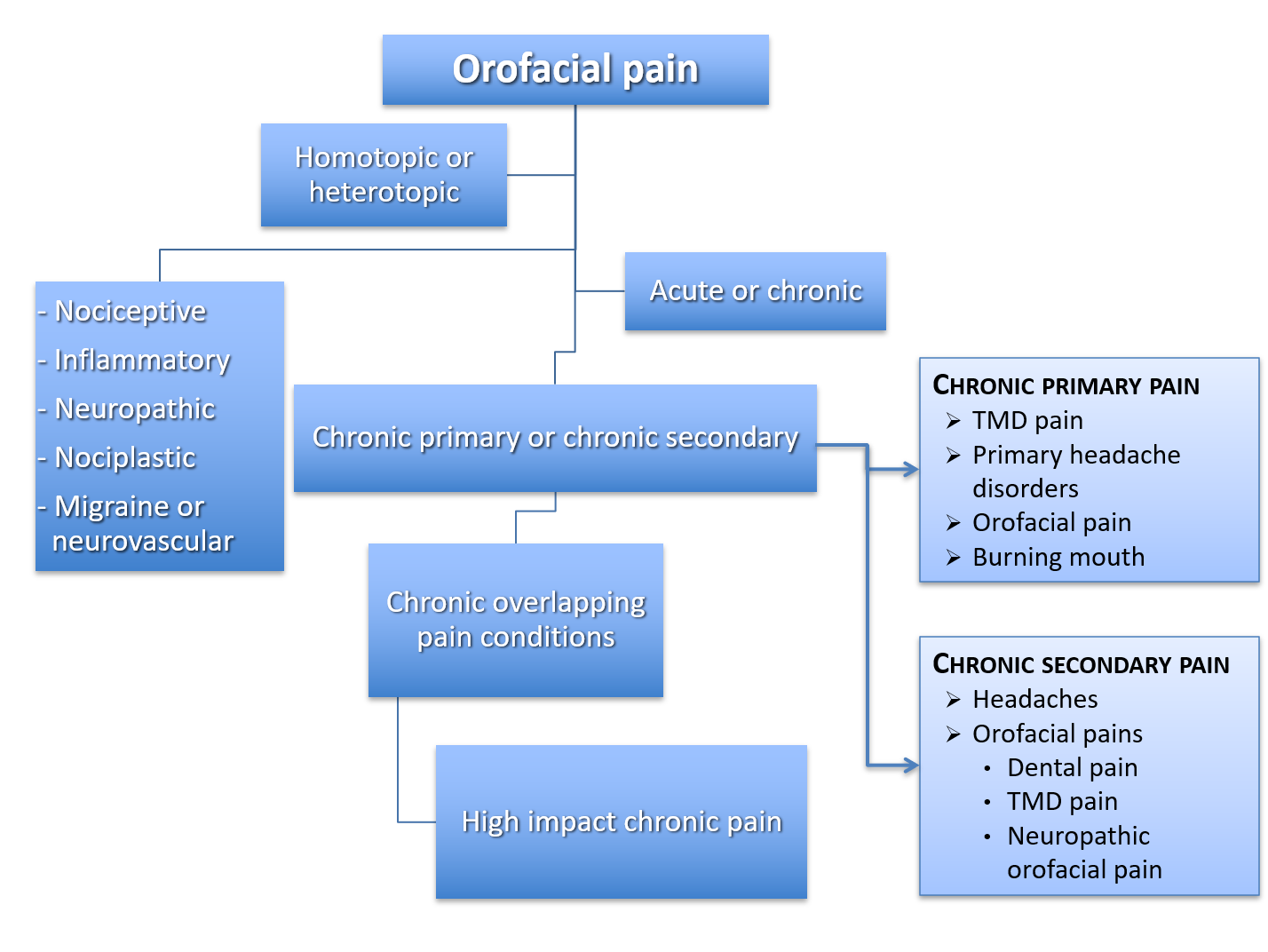
Figure 2: Summary of types of pain. Note: TMD = temporomandibular disorder.
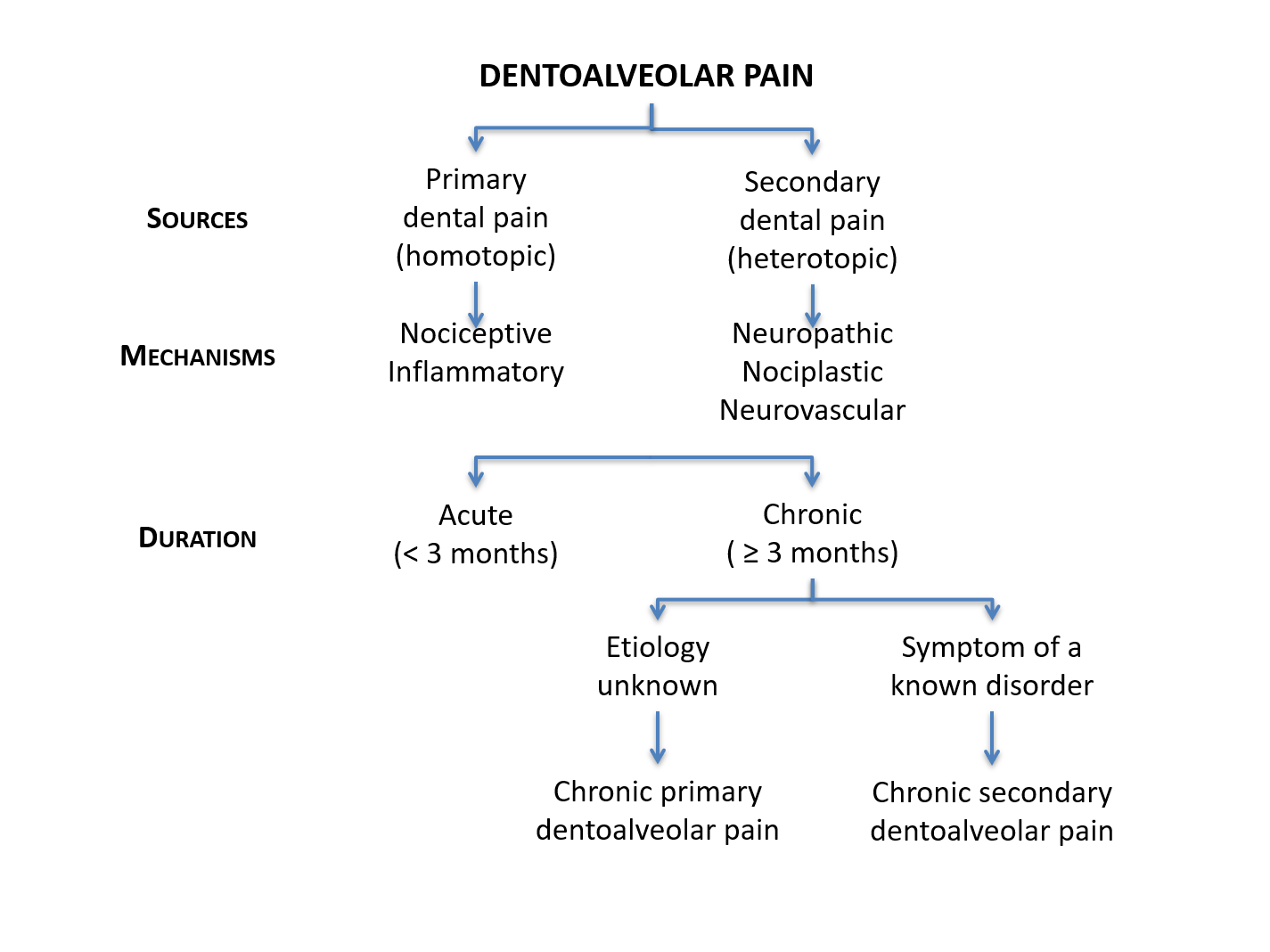
Figure 3: Summary of types of dentoalveolar pain.
The sensing of a noxious stimulus that arises from actual or threatened damage to non-neural tissue by activation of nociceptors (“nociceptive pain”) acts as a warning signal and is protective.3 An example is tooth pain induced by noxious stimuli (e.g., something hot or cold). The immune system, activated following tissue injury or infection, gives rise to an adaptive and protective “inflammatory pain,” a subtype of nociceptive pain that assists the healing process. Tissue damage triggers cellular release of inflammatory mediators that results in peripheral sensitization and hypersensitivity. This can create a situation that discourages physical contact and movement, thereby assisting healing of the injured body part.3 This type of pain is frequently seen by dentists in the form of symptomatic irreversible pulpitis.
“Neuropathic pain” occurs as a result of a lesion to neural tissue after nerve damage or disease of the peripheral or central somatosensory nervous system.3 One example is trigeminal neuralgia.
There is a further type of pain with no biologic role that suggests an altered nociceptive function despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system. This pain, which is neither nociceptive nor neuropathic, has recently been labeled “nociplastic pain” and represents a disorder of the nervous system of its own (e.g., fibromyalgia or nonspecific chronic low-back pain).4,5
Unique to the trigeminal area is a fifth type of pain with clinical features resembling primary headache pain and, more specifically, the trigeminal autonomic cephalalgias.6,7 This type of pain labeled “migraine or neurovascular” may cause toothache episodes or facial pain attacks mimicking cluster headaches.1
The term “mixed pain” has also emerged regarding pain states presenting with various combinations of nociceptive, neuropathic or nociplastic mechanisms acting simultaneously or concurrently in the same body area.8
Although such definitions might be an issue of semantics, these various pain mechanisms remain as descriptors and can help clinicians understand the patient’s pathophysiologic pain processes, thereby aiding in the establishment of a provisional working diagnosis and treatment plan.
Recognizing Chronic Dentoalveolar Pain
The paradigm of acute versus chronic pain based on the time elapsed since its onset has recently been challenged and considered arbitrary.9 Nonetheless, according to the International Classification of Diseases (ICD),10 any dentoalveolar pain lasting less than 3 months represents an acute condition. It becomes a chronic-pain problem when it persists or recurs beyond that period.11 Chronic pain in the orofacial area implies that it lasts at least 2 h/day on at least 50% of days over more than 3 months.12 Patients experiencing dentoalveolar or orofacial chronic pain are challenging for dentists unfamiliar with the concept of chronic pain as a complex multidimensional experience.
The ICD divides chronic pain into primary and secondary. The former refers to chronic-pain conditions of unknown etiology, and the latter represents a symptom secondary to an underlying disease.12 More specifically, chronic primary pain is “pain in one or more anatomical regions that 1) persists or recurs for longer than 3 months, 2) is associated with significant emotional distress (e.g., anxiety, anger, frustration, or depressed mood) and/or significant functional disability (interference in activities of daily life and participation in social roles), and 3) the symptoms are not better accounted for by another diagnosis.”12 Chronic primary pain can occur in any body system and body site, including the head and orofacial areas. Although an established cause cannot explain it, substantial knowledge may exist regarding pathophysiologic mechanisms.
Nicholas and co-authors12 and Benoliel and colleagues13 include the ICD list of chronic primary and secondary pain of the head and orofacial areas. The complexity of chronic pain is illustrated by the high degree of overlap or coprevalence of chronic-pain conditions with other commonly occurring pains, along with influences from biopsychosocial factors. Coexisting pain conditions are a well-recognized phenomenon called chronic overlapping pain conditions.14
Finally, one must not confound the words primary and secondary used in the ICD classification system with primary and secondary used to qualify the source of a dentoalveolar pain, where primary refers to a dental source (homotopic pain) and secondary refers to a nonodontogenic source (heterotopic pain; see below).
Common Pitfalls in the Diagnosis of Persistent Tooth Pain
Although differentiating the type of pain is of utmost importance, the significance of determining whether the site coincides with the source cannot be overemphasized. When there is no overlap, the pain is known as being heterotopic1 (as opposed to primary15 or homotopic16 when the site and source are in the same location). There are 3 kinds of heterotopic pain: central (arises from a lesion or event in the brain or brain stem and can include pain projected ipsilaterally to all 3 divisions of the trigeminal nerve17); projected (the pain sensation follows the course of a nerve branch that is irritated or triggered at some point on its anatomical trajectory); and referred pain (can include central sensitization).1,15,18
The failure of dentoalveolar pain to respond to multiple dental treatments suggests that another dental procedure is not likely to relieve it. A non-dental etiology should then be suspected, whether it is from a proximal or distal source. About 1 in 15–20 patients receiving endodontic treatment is at risk of persistent tooth pain despite adequate endodontic treatment,19,20 with at least half thought to have a nonodontogenic source.19 Nonodontogenic toothaches can result from a variety of disorders or conditions (Table 1), including musculoskeletal disorders of the masticatory or neck muscles, neurovascular/primary headache disorders, pathologic processes outside the immediate dentoalveolar or facial regions that refer or project pain to the teeth (e.g., cardiovascular disorders) and trigeminal neuropathic pain disorders.1,21,22
|
Causes of secondary dental pain |
Management |
|---|---|
|
*Possible neuropathic pain. |
|
Musculoskeletal
|
Physical therapy, cognitive behavioural therapy, trigger point injection or dry needling |
Primary headache disorders
|
Abortive therapy with drugs, such as triptans, calcium channel blockers |
Pathologic processes outside the immediate dentoalveolar or facial region that refer or project pain to the teeth
|
Disorder-specific drug therapy or interventions |
Neuropathic pain disorders
|
Pharmacologic treatment with drugs, such as anticonvulsants, selective serotonin reuptake inhibitors and other antidepressants; cognitive behavioural therapy |
Trigeminal neuropathic pain is a complex topic on its own because it can involve complex mechanisms such as peripheral and central sensitization. Conditions of particular interest, besides trigeminal neuralgia, are persistent idiopathic dentoalveolar pain (PIDAP), formerly called atypical odontalgia or phantom tooth pain22 (there is controversy as to whether PIDAP is a neuropathic pain23) and post-traumatic trigeminal neuropathic pain.22 Neuropathic orofacial pain can present as an episodic pain (e.g., short electrical or sharp and shooting pain that may be paroxysmal) or as a continuous burning, tingling, throbbing or dull aching pain that can be sharp at times.22 The main distinction between post-traumatic trigeminal neuropathic pain and persistent idiopathic dentoalveolar pain is a history of injury to the peripheral trigeminal nerve as a causative event for the former while there is no such event or local cause that can explain the latter.22
Special chairside tests used by specialists in secondary care centres may help uncover abnormal sensations, such as hypersensitivity or allodynia (positive signs) or decreased sensations or anesthesia (negative signs), in areas of persistent pain.24 Such tests include qualitative somatosensory tests at sites of reported intraoral pain in soft tissue locations (e.g., gingiva of the painful tooth, edentulous ridges where patients continue to perceive toothache-like pain following an extraction) using tactile stimuli (e.g., with a cotton swab) for sensitivity to light touch, pin-prick (e.g., with a toothpick, periodontal probe or dental explorer) for pain sensitivity and a cold stainless steel spatula for thermal sensation.24,25 Combined with history data and clinical findings, these tests may help uncover potential neuropathic pain conditions.
Therefore, before settling on a diagnosis, one has to ask what else this condition could be. While contemplating this important question, clinicians must be aware of cognitive dispositions to respond, or cognitive biases, of which there are at least 30.26 Overconfidence (a universal tendency to believe we know more than we do), the anchoring effect (the tendency to perceptually lock onto salient features in the patient’s initial presentation too early in the diagnostic process and failing to adjust this initial impression in light of later information) and availability bias (the disposition to judge things as being more likely, or frequently occurring, if they readily come to mind; e.g., recent experience with a disease may inflate the likelihood of its being diagnosed) are a few examples of factors that may be associated with diagnostic inaccuracies or suboptimal management.26,27 In addition, clinicians should be aware of “search satisfying” (the universal tendency to call off a search once something is found).26 This cognitive bias might cause a clinician to stop at the first diagnosis that seems to fit some of the signs and symptoms and their desire to find a condition that they can treat.
Roadmap for the Management of Persistent Tooth Pain
Considering the abovementioned complexities, diagnosing and managing persistent or recurrent tooth pain can be highly challenging. The task of not overlooking a dental cause relies on the general dentist’s competence to conduct a comprehensive toothache history, inquire about past treatments and perform a thorough clinical examination that includes chair-side testing and imaging studies. The International Classification of Orofacial Pain, which provides a comprehensive listing and diagnostic criteria for conditions and disorders causing primary dental pain, can assist clinicians with this crucial task.22,28 Referral to an endodontist is recommended when there is doubt about a persistent toothache from symptomatic irreversible pulpitis or failed endodontic treatment. The lack of evidence for a dental source should prompt the dentist to consider a secondary dental pain (heterotopic toothache) from non-dental sources (Table 1). Among the clues suggesting a non-dental persistent toothache are a history of multiple unsuccessful treatments, tooth pain persisting >3 months and ominous unusual presenting symptoms. Consultation with an oral medicine specialist is then recommended; however, confirming a provisional working diagnosis may require further diagnostic workup and referral to appropriate colleagues, such as oral and maxillofacial surgeons, physicians, ear/nose/throat specialists or neurologists. Kapos and colleagues21 provide an overview of conditions that may cause nonodontogenic toothaches.
Although treatment of secondary dentoalveolar pain varies according to the underlying condition (see Table 1 and Figure 4), recognizing factors that interfere with treatment response cannot be overlooked when they affect the patient’s quality of life. With that in mind, the most holistic approach to managing patients with chronic dentoalveolar pain is the biopsychosocial model that takes into account, aside from the physical Axis I diagnosis (based on the DC/TMD dual-axis assessment29), the patient’s cognitive, emotional and behavioural profiles. It makes appraising the psychosocial impact of the pain on the patient’s condition (Axis II29) imperative, as early recognition and management of psychological and social distresses (Axis II factors) can positively influence the course of a chronic-pain patient’s condition and optimize treatment responses.30
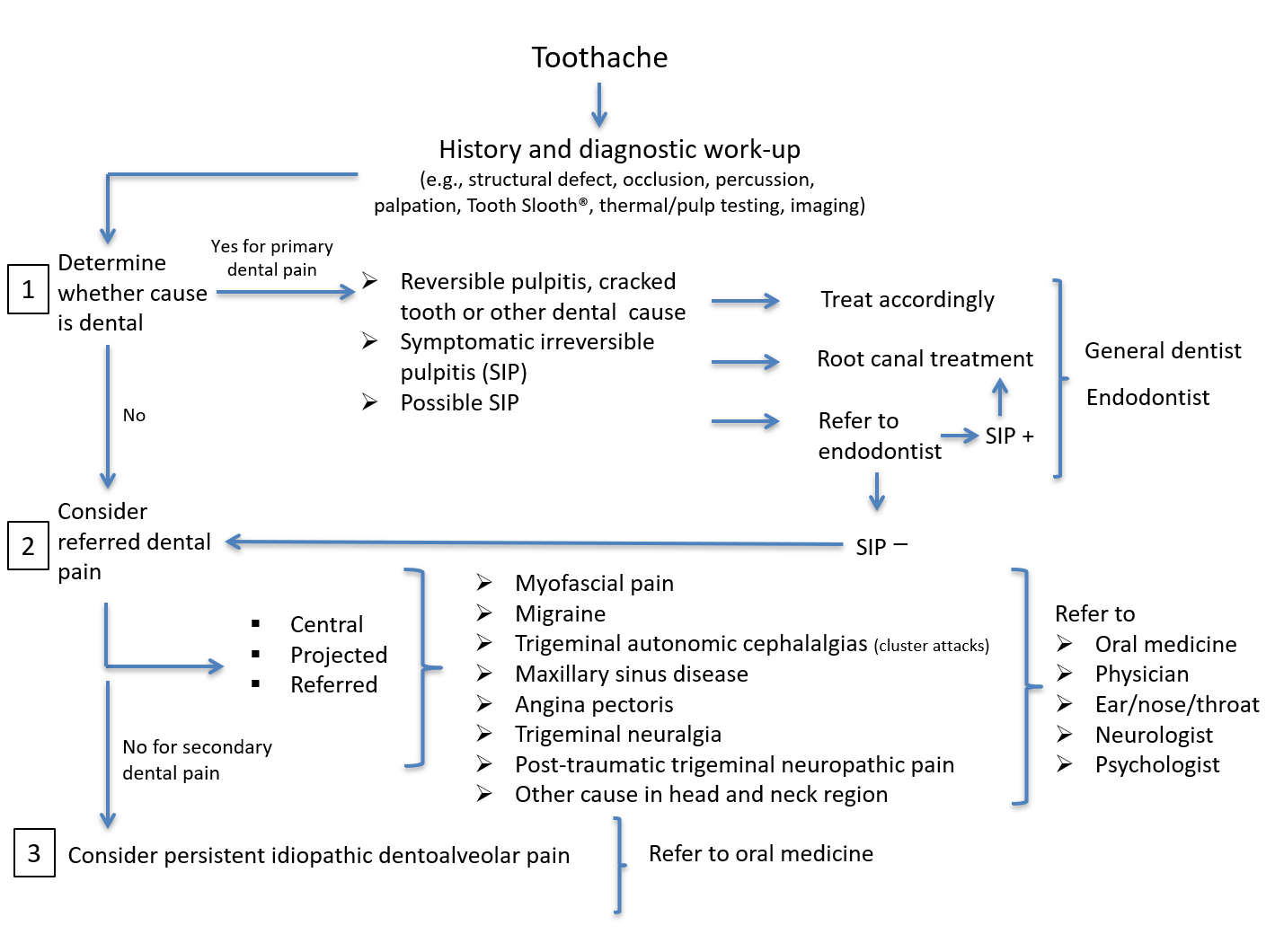
Figure 4: Workflow algorithm for patient with toothache.
Understanding that chronic-pain patients have both physical and psychosocial issues to a certain extent and, thus, knowing where a patient fits on each axis may influence treatment decisions. For example, some patients have predominantly Axis I components and only minimal psychosocial or Axis II involvement (e.g., many dental patients with acute pain whose anxiety abates after successful treatment), while others might have what appears to be little in the way of physical/clinical factors, but significant psychosocial involvement.31 Self-report of recurrent or persistent pain at other body sites is a clue that a patient might have a more complex chronic-pain condition categorized as high impact chronic pain, i.e., chronic pain that limits life or work activities on most days or every day for at least 6 months.32
Appraisal of a psychosocial Axis II patient’s profile is best when done concurrently with the comprehensive history by administering brief and widely used self-report screening instruments.33 These include a “pain drawing” that allows the patient to indicate on a body map the location and spreading of their tooth pain and any other site of pain they may be experiencing.34 The Graded Chronic Pain Scale (GCPS) assesses average, worst and current pain intensity and pain-related interference with daily activities,35 and the revised GCPS differentiates mild, bothersome and high-impact chronic pain.36 The Patient Health Questionnaire (PHQ-4) screens for anxiety and depressive symptoms.37 Questionnaires developed for clinic-setting assessment of psychosocial and behavioural factors and functional consequences of pain used by specialists in secondary care centres can be found elsewhere.38
Patients with persistent dentoalveolar pain in the absence of a dental cause, and more specifically patients with post-traumatic trigeminal neuropathic pain or persistent idiopathic dentoalveolar pain, require a multidisciplinary approach that addresses such comorbidities as depression, anxiety, somatic awareness, pain catastrophizing and poor sleep. This is even more crucial when the pain is disproportionate to the signs found in the area of the reported symptoms.39,40 Other factors, such as the patient’s trajectory in the health care system, which depends on their coping skills, attitude, self-constructed explanations, beliefs and how they deal with stress, may also need attention while the dentist is gaining the patient’s trust and being honest about what can be done and what should be expected despite the best treatment.38,40-42
Few chronic dentoalveolar pain patients can expect complete relief; however, dentists must be supportive and let patients know that their well-being can improve if they adhere to specific recommendations, although it will not come suddenly but progressively.43 Multidisciplinary approaches combining psychological support, behavioural strategies and selective medications acting on pain inhibitory mechanisms, central and peripheral sensitization and comorbidities may significantly reduce the pain and improve the quality of life of many chronic orofacial pain patients (Table 1).39
Conclusion
Patients with persistent dentoalveolar pain following adequate dental treatment are not “difficult” or “problem” patients, but rather complex patients, and clinicians must protect themselves from becoming “difficult” dentists when trying to help them.44 It is important to listen to these patients and make sure that they have time to tell their entire story.45 Finding out whether the patient’s overall presentation suggests that all, or at least most, of it can be rationally explained by a dental diagnosis alone is essential. However, there can be uncertainty for the dentist (and patient) when making decisions regarding diagnoses and treatments.46 If there is indecision, clinicians should not forge ahead with another dental treatment, but should consider other options (potentially including artificial intelligence to aid in the diagnosis of the orofacial pain condition).47 All patients with pain have Axis I and II components. Once pain becomes chronic, or perhaps sooner if the pain is more centrally mediated,9 Axis II issues can become more clinically significant.38 However, some patients are able to cope better than others under these circumstances — so-called “adaptive copers.”48 It is important to ensure that an Axis I condition primarily affecting the periphery (e.g., a tooth with symptomatic irreversible pulpitis) can be definitively diagnosed and to recognize significant Axis II components that have greater relevance to the management needs of the patient before beginning an irreversible dental treatment and acknowledge the possibility that a patient should be referred to a health care colleague who can provide appropriate management.
THE AUTHORS
Corresponding author: Dean Kolbinson, Dental Clinic, College of Dentistry, University of Saskatchewan, 105 Wiggins Rd, Saskatoon, SK S7N 5E4. Email: dean.kolbinson@usask.ca
The authors have no declared financial interests.
This article has been peer reviewed.
References
- De Laat A. Differential diagnosis of toothache to prevent erroneous and unnecessary dental treatment. J Oral Rehabil. 2020;47(6):775-81.
- Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161(9):1976-82.
- Vardeh D, Mannion RJ, Woolf CJ. Toward a mechanism-based approach to pain diagnosis. J Pain. 2016;17(9 Suppl):T50-69.
- Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice AS, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157(7):1382-6.
- Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. 2021;162(11):2629-34.
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211.
- Robert C, Bourgeais L, Arreto CD, Condes-Lara M, Noseda R, Jay T, et al. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. J Neurosci. 2013;33(20):8827-40.
- Freynhagen R, Parada HA, Calderon-Ospina CA, Chen J, Emril DR, Fernandez-Villacorta FJ, et al. Current understanding of the mixed pain concept: a brief narrative review. Curr Med Res Opin. 2019;35(6):1011-8.
- Loeser JD. A new way of thinking about pains. Pain. 2022;163(9):1670-4.
- MG31 Acute Pain. ICD-11 for mortality and morbidity statistics (v. 01/2023). Available: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1404135736 (accessed 2023 March 2).
- MG30 Chronic Pain. ICD-11 for mortality and morbidity statistics (v. 01/2023). Available: https://icd.who.int/browse11/l-m/en#/http://id.who.int/icd/entity/1581976053 (accessed 2023 March 2).
- Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28-37.
- Benoliel R, Svensson P, Evers S, Wang SJ, Barke A, Korwisi B, et al. The IASP classification of chronic pain for ICD-11: chronic secondary headache or orofacial pain. Pain. 2019;160(1):60-8.
- Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD. Overlapping chronic pain conditions: implications for diagnosis and classification. J Pain. 2016;17(9 Suppl):T93-107.
- Okeson JP. The central processing of pain. In: Okeson JP. Bell’s oral and facial pain, 7th edition. Hanover Park: Quintessence Publishing, Inc.; 2014. p.71-91.
- Homotopic. Merriam-Webster.com medical dictionary. Springfield, Mass.: Merriam-Webster; 2022. Available: https://www.merriam-webster.com/medical/homotopic (accessed 2023 March 2).
- Fontaine D, Almairac F, Santucci S, Fernandez C, Dallel R, Pallud J, et al. Dural and pial pain-sensitive structures in humans: new inputs from awake craniotomies. Brain. 2018;141(4):1040-8.
- Chichorro JG, Porreca F, Sessle B. Mechanisms of craniofacial pain. Cephalalgia. 2017;37(7):613-26.
- Nixdorf DR, Moana-Filho EJ, Law AS, McGuire LA, Hodges JS, John MT. Frequency of nonodontogenic pain after endodontic therapy: a systematic review and meta-analysis. J Endod. 2010;36(9):1494-8.
- Nixdorf DR, Moana-Filho EJ, Law AS, McGuire LA, Hodges JS, John MT. Frequency of persistent tooth pain after root canal therapy: a systematic review and meta-analysis. J Endod. 2010;36(2):224-30.
- Kapos FP, Nixdorf DR. Non-odontogenic “tooth” pain. In: Ferreira JNAR, Fricton J, Rhodus N, editors. Orofacial disorders: current therapies in orofacial pain and oral medicine. Cham, Switzerland: Springer International Publishing AG; 2017. p.197-211.
- International classification of orofacial pain, 1st edition (ICOP). Cephalalgia. 2020;40(2):129-221.
- Malacarne A, Spierings ELH, Lu C, Maloney GE. Persistent dentoalveolar pain disorder: a comprehensive review. J Endod. 2018;44(2):206-11.
- Costa YM, Bonjardim LR, Conti PCR, Svensson P. Psychophysical evaluation of somatosensory function in oro-facial pain: achievements and challenges. J Oral Rehabil. 2021;48(9):1066-76.
- Svensson P, Baad-Hansen L, Pigg M, List T, Eliav E, Ettlin D, et al. Guidelines and recommendations for assessment of somatosensory function in oro-facial pain conditions — a taskforce report. J Oral Rehabil. 2011;38(5):366-94.
- Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78(8):775-80.
- Saposnik G, Redelmeier D, Ruff CC, Tobler PN. Cognitive biases associated with medical decisions: a systematic review. BMC Med Inform Decis Mak. 2016;16(1):138.
- Pigg M, Nixdorf DR, Law AS, Renton T, Sharav Y, Baad-Hansen L, et al. New international classification of orofacial pain: what is in it for endodontists? J Endod. 2021;47(3):345-57.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial Pain Headache. 2014;28(1):6-27.
- Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. Risk factors for onset of chronic oro-facial pain — results of the North Cheshire oro-facial pain prospective population study. Pain. 2010;149(2):354-9.
- Dworkin SF. Psychosocial impact of orofacial pain. In: Turp JC, Sommer C, Hugger A, editors. The puzzle of orofacial pain: integrating research into clinical management, volume 15. Basel, Switzerland: S. Karger AG; 2007, p.187-208.
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-6.
- Visscher CM, Baad-Hansen L, Durham J, Goulet JP, Michelotti A, Barraza CR, et al. Benefits of implementing pain-related disability and psychological assessment in dental practice for patients with temporomandibular pain and other oral health conditions. J Am Dent Assoc. 2018;149(6):422-31.
- Pain drawing, v. 12. International Network for Orofacial Pain and Related Disorders Methodology; 2013. Available: https://ubwp.buffalo.edu/rdc-tmdinternational/wp-content/uploads/sites/58/2017/01/DC-TMD-pain-drawing_2013_05_12.pdf (accessed 2023 March 2).
- Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133-49.
- Von Korff M, DeBar LL, Krebs EE, Kerns RD, Deyo RA, Keefe FJ. Graded chronic pain scale revised: mild, bothersome, and high impact chronic pain. Pain. 2020;161(3):651-61.
- Kroenke K, Spitzer RL, Williams JBW, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613-21.
- Turk DC, Fillingim RB, Ohrbach R, Patel KV. Assessment of psychosocial and functional impact of chronic pain. J Pain. 2016;17(9 Suppl):T21-49.
- Zakrzewska JM. Differential diagnosis of facial pain and guidelines for management. Br J Anaesth. 2013;111(1):95-104.
- Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD. The role of psychosocial processes in the development and maintenance of chronic pain. J Pain. 2016;17(9 Suppl):T70-92.
- Bonathan CJ, Zakrzewska JM, Love J, Williams AC. Beliefs and distress about orofacial pain: patient journey through a specialist pain consultation. J Oral Facial Pain Headache. 2014;28(3):223-32.
- Nilsson IM, Willman A. Treatment seeking and self-constructed explanations of pain and pain management strategies among adolescents with temporomandibular disorder pain. J Oral Facial Pain Headache. 2016;30(2):127-33.
- Pigg M, Svensson P, Drangsholt M, List T. Seven-year follow-up of patients diagnosed with atypical odontalgia: a prospective study. J Orofac Pain. 2013;27(2):151-64.
- Türp JC. The “problematic patient”: what is the problem? Illustrated by the example of temporomandibular disorders. Dtsch Zahnarztl Z Int. 2021;3(1):16-24. Available: https://www.online-dzz.com/fileadmin/user_upload/OA_Problematic_patient_01.pdf (accessed 2023 Dec. 3).
- Häggman-Henrikson B, Lobbezoo F, Durham J, Peck C, List T. The voice of the patient in orofacial pain management. J Evid Based Dent Pract. 2022;22(1S):101648.
- Pigg M, Brodén J, Fransson H, EndoReCo, the Foresight Research Consortium, Vareman N. How do we and how should we deal with uncertainty in endodontics? Int Endod J. 2022;55(4):282-9.
- Kreiner M, Viloria J. A novel artificial neural network for the diagnosis of orofacial pain and temporomandibular disorders. J Oral Rehabil. 2022;49(9):884-9.
- Rudy TE, Turk DC, Zaki HS, Curtin HD. An empirical taxometric alternative to traditional classification of temporomandibular disorders. Pain. 1989;36(3):311-20.



