ABSTRACT
Objective: To evaluate the effectiveness of various bioimplants used for augmentation of the maxillary sinus floor by means of a rabbit model.
Materials and Methods: Bone was harvested from the posterior iliac crest of 40 adult New Zealand white rabbits to allow bilateral augmentation of the floor of the maxillary sinus with autogenous bone or other materials. One of the following was grafted to the maxillary sinus of each rabbit: particulated autogenous bone, demineralized bone matrix (DBM), DBM combined with purified bone morphogenetic protein (BMP–DBM bioimplants) and bioimplants consisting of a poloxamer gel with BMP in 1 of 2 different doses. Animals were sacrificed at 2 or 8 weeks. Histologic examination was used to assess biologic healing in the various samples. Histomorphometry was used to demonstrate and quantify bone formation.
Results: After 2 weeks, the BMP-containing bioimplants had produced more new bone than any of the other materials. Particulated autogenous bone grafts produced less new bone initially (after 2 weeks), but the amount of bone produced by these grafts gradually increased, to levels comparable to the BMP-containing bioimplants by 8 weeks. For groups in which the poloxamer gel was used as a carrier for BMP or where BMP was used in combination with DBM, the amount of bone generated by 8 weeks was similar to that produced by autogenous bone.
Conclusion: The rabbit maxillary sinus model allowed evaluation of multiple types of bioimplants that could be suitable for peri-implant maxillary reconstruction. BMP-containing bioimplants demonstrated promise as alternatives to autogenous bone grafts for sinus-augmentation procedures. These bioimplants had more rapid initial bone production than all other materials, including autogenous bone. In the future, such biomaterials may enable earlier placement of dental implants into augmented maxillary sinuses.
Introduction
The maxillary sinus is surrounded by highly vascular tissue,1 making it an ideal site to receive a bone graft or, in the future, tissue-engineered constructs.2 Autogenous bone grafts are considered the gold standard for the repair of most osseous defects,3,4 including augmentation of the maxillary sinus. However, there are limitations to the amount of bone that can be harvested from an individual human skeleton for autogenous grafting. In addition, autogenous bone grafting is associated with a certain degree of morbidity at the harvesting site, including infection and pain, which may increase the length of the hospital stay.5,6 As a result, there has been interest in developing allografts, xenografts, synthetic bioimplants and cellularized constructs for reconstructive procedures involving bone. Numerous studies have compared the effectiveness of these alternatives as potential replacements for autogenous bone grafts.7-15
Bone allografts, such as demineralized bone matrix (DBM), were first used to reconstruct skull defects in dogs more than 100 years ago.12,13 Urist14 described heterotopic bone formation after implantation of DBM into mouse muscle pouches, and researchers then seriously considered DBM as a potential bioimplant for osseous repair.14 DBM also contains noncollagenous proteins (NCPs), which may be important markers in bone formation.
Given the constraints of both autogenous bone grafts and bone allografts, synthetic bioimplants containing bone morphogenetic proteins (BMPs) have garnered interest as being potentially suitable for osseous repair in clinical settings. Of the many BMPs that have been isolated, all but one are members of the transforming growth factor β (TGF-β) superfamily,16,17 the exception being BMP-1. BMPs induce pluripotent mesenchymal stem cells to differentiate into bone-forming osteoblasts.18 To date, BMP-2, BMP-4 and BMP-7 have all been shown to stimulate de novo, in vitro and in vivo bone formation in various animal models.5,18,19 Recombinant technology has been used to produce synthetic BMP-7 (OP-1, Stryker, Allendale, NJ) and BMP-2 (Infuse, Medtronic, Fridley, MN), both of which are available for clinical use.5
This study investigated the effectiveness of synthetic bioimplants for augmentation of the maxillary sinus. Autogenous bone was used as the gold standard (control), and bioimplants containing different concentrations of BMP were investigated and compared with DBM.
Materials and Methods
The protocol for the study was approved by the University of Toronto Animal Care and Ethics Committee (protocol 20003105). Forty adult male New Zealand white rabbits (Charles River Laboratories, Wilmington, MA), weighing 3.5 to 4 kg, were randomly divided into 2 groups of 20 animals each, one designated to undergo sacrifice at 2 weeks and the other to undergo sacrifice at 8 weeks. A veterinarian induced general anesthesia, and bone was harvested from the right posterior iliac crest of each animal. A maxillary sinus lift procedure was then performed using an extraoral lateral-window approach (Fig. 1). A round diamond bur was used to outline the lateral wall of the maxillary sinus, and the sinus membrane was then elevated from the sinus floor. One of 5 possible materials was placed onto the sinus floor:
- autogenous bone graft from the posterior iliac crest (Fig. 2)
- 10 mg of BMP 7–NCP in a poloxamer carrier (low-dose BMP)
- 25 mg of BMP 7–NCP in a poloxamer carrier (high-dose BMP)
- allograft DBM in a poloxamer carrier (prepared from rabbit long bones, as described below, and referred to hereafter as “DBM”)
- 10 mg of BMP 7–NCP combined with DBM in a poloxamer carrier (BMP–DBM).
The poloxamer F-127 (BASF Corp., Parsippany, NJ) is a hydrophobic copolymer that is a liquid at room temperature and a gel at body temperature.20 It is therefore suitable for use as a delivery system for BMP or other growth factors.
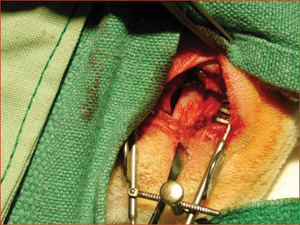 Figure 1: Maxillary sinus of a rabbit exposed bilaterally to receive bone graft or bioimplant.
Figure 1: Maxillary sinus of a rabbit exposed bilaterally to receive bone graft or bioimplant.
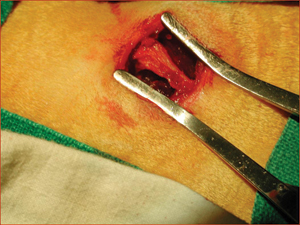 Figure 2: Posterior iliac crest of a rabbit exposed for harvesting of material for autogenous bone grafting.
Figure 2: Posterior iliac crest of a rabbit exposed for harvesting of material for autogenous bone grafting.
The 5 treatments were randomly assigned within each of the 2 experimental groups, without regard to the side of implantation. Following sacrifice by the veterinarian, after 2 or 8 weeks, the maxillae of each rabbit were dissected free from the cranium and fixed in 10% paraformaldehyde. The specimens were then decalcified with 45% formic acid containing 0.2 mol/L sodium citrate before final processing for histologic examination.
Preparation of Rabbit DBM
The long bones of New Zealand white rabbits were collected, along with the soft tissues, and the epiphyses were removed. A hand instrument was then used to cut the bones into 1.5-cm strips before freezing with liquid nitrogen. After freezing, the bones were mechanically ground to a particle size of 1 mm3. The particulated ground bone was sterilized, and the fat was removed by washing overnight with a 1:1 chloroform–methanol mixture at room temperature. The powder was allowed to air-dry before decalcification with 0.6 mol/L hydrochloric acid at 4°C for 24 hours, followed by freezing with liquid nitrogen and lyophilization.
Qualitative Analysis: Histology
Histologic sections of the specimens of maxillary sinus were created by sectioning through the midpoint of the defect and then embedding in paraffin. Multiple 6-µm sections were cut with a microtome and stained with hematoxylin and eosin for conventional light microscopy.
Quantitative Analysis: Histomorphometry
Histomorphometric analysis was performed by evaluating the stained sections under a light microscope (Leitz, Wetzlar, Germany) at 4× magnification. Images were captured using a digital image-acquisition device (RT Color, Diagnostic Instruments Inc., Sterling Heights, MI). The cells and newly formed bone in each image were quantified with Image Pro Plus 4.1 software (Media Cybemetics, Silver Spring, MD). Calibration was performed using a slide calibrated in micrometres, and the setting remained unchanged during the analysis of all samples.
Statistical Analysis
Histomorphometric results were analyzed using SPSS version 10.0 (SPSS Science Inc., Chicago, IL). One-way analysis of variance (ANOVA) was used for all within-group and between-group comparisons. Post hoc Bonferroni tests were also performed to evaluate the statistical significance of differences between groups of bioimplants. Statistical significance was set at p < 0.05.
Results
Histologic Analysis
After 2 Weeks
After 2 weeks, grafted mature cortical bone fragments were present in the rabbit maxillary sinuses augmented with autogenous bone grafts (Fig. 3a). Few lacunae within the grafted bone were occupied by osteocytes (Fig. 3b). The margins of the grafted particles demonstrated active bone turnover and remodelling. Resorption of the grafted mature cortical bone was coupled with areas of newly formed woven bone. The new bony regenerate demonstrated plump osteocytes and osteoblasts.
Sections from rabbits that received bioimplants containing DBM in a poloxamer carrier had residual particles of implanted allograft (Fig. 4a). These particles were larger than those of the autograft. None of the lacunae within the allograft were occupied by osteocytes (Fig. 4b). The margins of the implanted allograft also demonstrated active bone turnover and remodelling. As with the autograft, new bony regenerate was present within resorbed regions of the allograft, as well as between allograft particles.
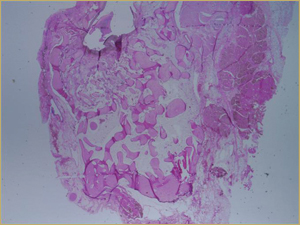 Figure 3a: Low-power histologic examination of rabbit maxillary sinus augmented with autogenous bone graft at 2 weeks after grafting. Hematoxylin and eosin (H&E) stain, 50× magnification.
Figure 3a: Low-power histologic examination of rabbit maxillary sinus augmented with autogenous bone graft at 2 weeks after grafting. Hematoxylin and eosin (H&E) stain, 50× magnification.
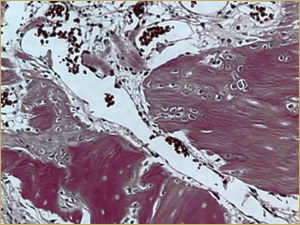 Figure 3b: High-power histologic examination of rabbit maxillary sinus augmented with autogenous bone graft at 2 weeks after grafting. H&E stain, 200× magnification.
Figure 3b: High-power histologic examination of rabbit maxillary sinus augmented with autogenous bone graft at 2 weeks after grafting. H&E stain, 200× magnification.
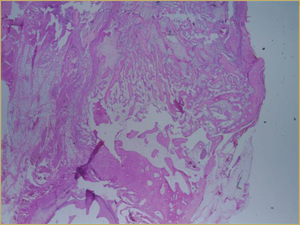 Figure 4a: Low-power histologic examination of rabbit maxillary sinus augmented with demineralized bone matrix at 2 weeks after procedure. H&E stain, 50× magnification.
Figure 4a: Low-power histologic examination of rabbit maxillary sinus augmented with demineralized bone matrix at 2 weeks after procedure. H&E stain, 50× magnification.
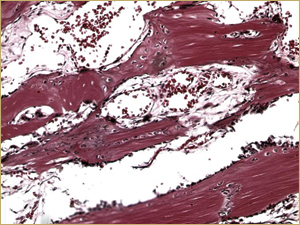 Figure 4b: High-power histologic examination of rabbit maxillary sinus augmented with demineralized bone matrix at 2 weeks after procedure. H&E stain, 200× magnification.
Figure 4b: High-power histologic examination of rabbit maxillary sinus augmented with demineralized bone matrix at 2 weeks after procedure. H&E stain, 200× magnification.
There was no difference in appearance between sinuses augmented with low-dose (10 mg) BMP bioimplants (Fig. 5a) and those augmented with high-dose (25 mg) BMP bioimplants (Fig. 5b). Both groups had a high concentration of thin, woven bony trabeculae, which were smaller and more numerous than in sinuses with either autografts or DBM bioimplants.
Bioimplants containing both DBM and 10 mg of BMP in the poloxamer carrier yielded a histologic picture with a mixture of characteristics. Large implanted particles of mature lamellar bone were undergoing remodelling (Fig. 6a), similar to the findings with the DBM-only bioimplants. In addition, the entire bioimplanted area contained small-diameter woven bony trabeculae surrounded by highly vascular connective tissue, with the exception of the region occupied by the DBM (Fig. 6b), similar to the findings for the BMP-only implants.
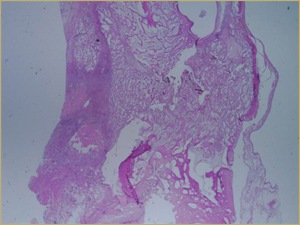 Figure 5a: Low-power histologic examination of rabbit maxillary sinus augmented with low-dose (10 mg) bone morphogenetic protein at 2 weeks after procedure. H&E stain, 50× magnification.
Figure 5a: Low-power histologic examination of rabbit maxillary sinus augmented with low-dose (10 mg) bone morphogenetic protein at 2 weeks after procedure. H&E stain, 50× magnification.
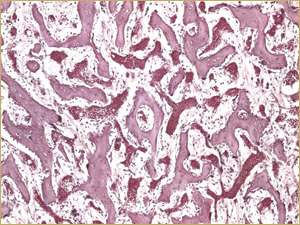 Figure 5b: High-power histologic examination of rabbit maxillary sinus augmented with high-dose (25 mg) bone morphogenetic protein at 2 weeks after procedure. H&E stain, 200× magnification.
Figure 5b: High-power histologic examination of rabbit maxillary sinus augmented with high-dose (25 mg) bone morphogenetic protein at 2 weeks after procedure. H&E stain, 200× magnification.
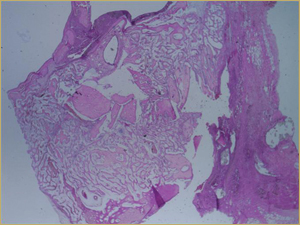 Figure 6a: Low-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein in demineralized bone matrix at 2 weeks after procedure. H&E stain, 50× magnification.
Figure 6a: Low-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein in demineralized bone matrix at 2 weeks after procedure. H&E stain, 50× magnification.
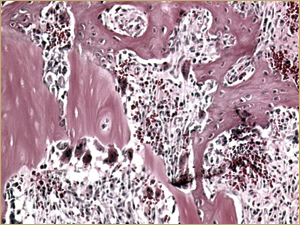 Figure 6b: High-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein in demineralized bone matrix at 2 weeks after procedure. H&E stain, 200× magnification.
Figure 6b: High-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein in demineralized bone matrix at 2 weeks after procedure. H&E stain, 200× magnification.
After 8 Weeks
By 8 weeks, all of the sinuses appeared histologically similar (Figs. 7–10). The mature lamellar bone that had been grafted in the autogenous bone group had been remodelled and was no longer present. Similarly, the numerous small-diameter woven bony trabeculae seen at 2 weeks in the BMP groups were no longer present. The sinuses all contained mature, small-diameter lamellar bony trabeculae. No qualitative histologic differences were apparent among the 5 treatment groups at 8 weeks (Figs. 7–10).
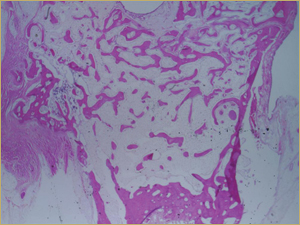 Figure 7: Low-power histologic examination of rabbit maxillary sinus augmented with autogenous bone graft at 8 weeks after procedure. H&E stain, 100× magnification.
Figure 7: Low-power histologic examination of rabbit maxillary sinus augmented with autogenous bone graft at 8 weeks after procedure. H&E stain, 100× magnification.
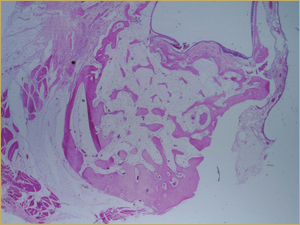 Figure 8: Low-power histologic examination of rabbit maxillary sinus augmented with demineralized bone matrix at 8 weeks after procedure. H&E stain, 100× magnification.
Figure 8: Low-power histologic examination of rabbit maxillary sinus augmented with demineralized bone matrix at 8 weeks after procedure. H&E stain, 100× magnification.
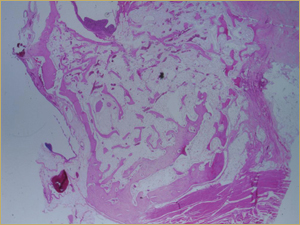 Figure 9: Low-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein at 8 weeks after procedure. H&E stain, 100× magnification.
Figure 9: Low-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein at 8 weeks after procedure. H&E stain, 100× magnification.
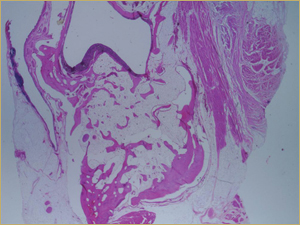 Figure 10: Low-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein in demineralized bone matrix at 8 weeks after procedure. H&E stain, 100× magnification.
Figure 10: Low-power histologic examination of rabbit maxillary sinus augmented with bone morphogenetic protein in demineralized bone matrix at 8 weeks after procedure. H&E stain, 100× magnification.
Histomorphometric Analysis
After 2 Weeks
In the histomorphometric analysis, the first parameter quantified was total bone area, which was calculated as a percentage of the grafted region. At 2 weeks, the total bone area for the autograft was 37.7% ± 7.2%, a value not statistically significant from total bone area for other bioimplants (p = 0.38).
One-way ANOVA of percentage total bone area, total trabecular surface area and average total trabecular size demonstrated no significant differences among the various bioimplants at 2 weeks after the procedures. The results of post hoc Bonferroni tests supported this result, demonstrating no significant differences in multiple comparisons among the bioimplants (p > 0.05).
In contrast, one-way ANOVA comparing the bioimplants in terms of percentage of new bone area at 2 weeks revealed significant differences in both area of new bone and new trabecular surface area. However, no significant differences were identified with regard to average new trabecular size. Post hoc Bonferroni tests showed that at 2 weeks, the bioimplants containing BMP formed more new bone and had more new bony surface area than those without BMP (p < 0.01).
In addition, of all the bioimplants, those containing BMP produced the highest ratio of new bone to existing bone after 2 weeks.
After 8 Weeks
As was the case at 2 weeks, one-way ANOVA of percentage total bone area, total trabecular surface area and average total trabecular size demonstrated no significant differences among the various bioimplants at 8 weeks after the procedures. The results of post hoc Bonferroni tests supported this result, demonstrating no significant differences in multiple comparisons among the bioimplants (p > 0.05).
Intragroup Results
One-way ANOVA comparing percentage new bone area, new trabecular surface area and average new trabecular size within the autograft group at 2 and 8 weeks demonstrated a significant increase in new bone area and new bone surface area (calculated as total new bone perimeter divided by implanted area) from 2 to 8 weeks, combined with a significant decrease in the average size of new trabeculae over the same period (both p < 0.05).
One-way ANOVA comparing percentage new bone area, new trabecular surface area and average new trabecular size within the high-dose and low-dose BMP groups at 2 and 8 weeks revealed a significant decrease in both new bone area and new bone surface area from 2 to 8 weeks (p < 0.05).
One-way ANOVA comparing percentage new bone area, new trabecular surface area and average new trabecular size within the BMP–DBM group at 2 and 8 weeks demonstrated a significant decrease in new bone surface area and a significant decrease in average new trabecular size (p < 0.05).
Finally, one-way ANOVA comparing percentage new bone area, new trabecular surface area and average new trabecular size within the DBM group at 2 and 8 weeks demonstrated no statistically significant differences (p > 0.05).
Discussion
Augmentation of the maxillary sinus is a clinically useful technique to augment severely pneumatized maxillary sinuses before reconstruction with a dental implant. The unique anatomy and physiology of the maxillary sinus makes it an ideal site to investigate bioimplants in a clinically relevant anatomic location.1,2
Detailed histologic and histomorphometric studies involving the entire maxillary sinus are impossible in human patients, and validated animal models are therefore required for such work. Animal models of the maxillary sinus involve membranous bones and are potentially more relevant for the study of craniofacial reconstruction than are bone-healing models involving more distant anatomic sites, such as the endochondral long bones. The goat21 and rabbit22 models have been used to investigate maxillary sinus augmentation procedures. In both mammals, the anatomy of the maxillary sinus is comparable to that of humans, and bone has been produced in the sinus floor using bioimplants.22-25
Histologic results obtained in the current investigation further validate use of the rabbit model for studies of maxillary sinus augmentation. All of the samples revealed early active bone formation (at 2 weeks). Autografted samples had new bone in the vicinity of grafted cortical bone particles, which suggests the osteoconductive capabilities of autografts. When the grafted region was viewed as a whole, new bone formation seemed to be greatest in the areas with grafted cortical bone, whereas there was less new bone in areas where grafted bone was absent.
Evidence of osteoinduction was found in the 2-week DBM samples, with new bone having been formed within the DBM and in areas in contact with this material. The distribution of new bone in close proximity to DBM indicates an osteoinductive effect.
The histologic appearance of bioimplants containing poloxamer combined with a low or high dose of BMP was similar, with smaller-diameter woven bony trabeculae. The BMP and poloxamer carrier were thoroughly mixed before implantation to aid in the homogenous distribution of the BMP. This effort appeared to be successful, given that the resulting histologic pattern showed uniform distribution of new woven bone in the bioimplanted area. The clustered distribution of new bone formation seen with autografting and with DBM bioimplants was absent from the BMP samples.
No qualitative differences were observed between autografts and DBM bioimplants at 8 weeks. Bone remodelling had taken place, the woven bone that was in contact with the graft particles had converted into lamellar trabeculae, and the stroma had changed into fatty marrow. The differences between BMP implants and non-BMP implants observed at 2 weeks had disappeared by 8 weeks.
When the value for new bone area was isolated from cortical bone in the autograft, DBM and BMP groups, it was determined that BMP-containing bioimplants (low-dose BMP, high-dose BMP and BMP–DBM) produced significantly more new bone than autografts or DBM alone (p < 0.01). The new woven bone was much more cellular than the grafted lamellar bone fragments, which often had mostly empty lacunae. Post hoc Bonferroni tests indicated that DBM alone yielded results not significantly different from the results of autografting.
For new bone surface area, a significant difference among bioimplants was found at 2 weeks, whereas the materials did not differ in total bone surface area (p < 0.01). New bone surface area is an estimation of the amount of surface area of new bone that is available to make contact with an osseointegrated implant placed into the augmented sinus.
Intergroup comparisons were also carried out at 8 weeks. At that point, no remnants of grafted or implanted cortical bone were seen in any of the augmented regions of the sinuses, and all of the sinuses had remodelled thoroughly. One-way ANOVA showed no significant differences (p > 0.05) between the groups in terms of total bone area, total bone surface area and average trabecular size within the bioimplanted region. This result supports the findings of other authors who have used the rabbit model for sinus augmentation studies, who also found no significant differences in the amounts of bone present at 8 weeks, regardless of the bioimplant used.22,25,26 This model had one major drawback, the assumption that 8 weeks of healing in the rabbit represents the ideal time for sinus remodelling before placement of the osseointegrated implant.
Intragroup comparisons of autografts at 2 and 8 weeks showed that the amount of new bone increased significantly over time (p < 0.05), with an increase in the amount of new bone surface area (p < 0.05) and a decrease in trabecular size (p < 0.05). These changes indicate resorption of the autograft and production of newly woven bone that increased in amount and maturity by 8 weeks.
BMP-containing bioimplants produced more new bone and greater new bone surface area at 2 weeks than autografts, but the advantage of these bioimplants seemed to be lost from 2 to 8 weeks, as the differences between the bioimplants and the autografts disappeared at 8 weeks. The trabecular size of the new regenerate was small at 2 weeks, and the regenerate resembled lamellar bone in the sinuses at 8 weeks. One-way ANOVA demonstrated decreases in the amount of new bone (p < 0.05) and the amount of new bone surface area (p < 0.05) with both high-dose and low-dose BMP bioimplants. Trabecular size did not change significantly from 2 to 8 weeks. This suggests that there is a period during which the sinuses augmented with BMP bioimplants have large numbers of new bone trabeculae, which provide increased surface area for osseointegration relative to autografts. This may offer additional stability to an implant earlier during the healing period.
Although the presence of BMP altered the rapidity of bone healing in the current study, other preparations containing platelet-derived growth factor, such as platelet-rich plasma, have not had a direct stimulatory effect on the healing of autogenous bone grafts in the same rabbit sinus model.27
Conclusion
BMP-containing bioimplants produced the greatest amount of new bone, and this new bone was similar in terms of trabecular size to the final state of the sinus at 8 weeks. Autografting produced less new bone initially (at 2 weeks), but the amount of new bone gradually increased to 8 weeks. Poloxamer was efficacious as a carrier for BMP, and by 8 weeks this combination was able to generate as much bone as an autogenous bone graft.
Endosseous implants placed in the sinuses and augmented with particulated autogenous bone have yielded predictable long-term results.28 BMP-containing bioimplants may become promising alternatives to autografts in sinus-augmentation procedures, as they result in more rapid bone formation.28-31 This may enable clinicians to place osseointegrated implants into augmented maxillae earlier after grafting and allow the patient to return to function sooner. Further studies are required to determine the effect of BMP and other growth factors on bone formation and implant survival in the maxillary sinus.
THE AUTHORS
References
- Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38(8):613-6.
- Tatum H Jr. Maxillary and sinus implant reconstructions. Dent Clin North Am. 1986;30(2): 207-29.
- Jensen OT, Shulman LB, Block MS, Iacono VJ. Report of the Sinus Consensus Conference of 1996. Int J Oral Maxillofac Implants. 1998;13(Suppl):11-45.
- Marx RE. Clinical application of bone biology to mandibular and maxillary reconstruction. Clin Plast Surg. 1994;21(3):377-92.
- Lane JM, Yasko AW, Tomin E, Cole BJ, Waller S, Browne M, et al. Bone marrow and recombinant human bone morphogenetic protein-2 in osseous repair. Clin Orthop Relat Res. 1999;361:216-27.
- Sandor GK, Rittenberg BN, Clokie CM, Caminiti MF. Clinical success in harvesting autogenous bone using a minimally invasive trephine. J Oral Maxillofac Surg. 2003;61(2):164-8.
- Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;174:28-42.
- Connolly J, Guse R, Lippiello L, Dehne R. Development of an osteogenic bone-marrow preparation. J Bone Joint Surg Am. 1989;71(5):684-91.
- Burwell RG. Studies in the transplantation of bone. VII. The fresh composite homograft-autograft of cancellous bone: an analysis of factors leading to osteogenesis in marrow transplants and in marrow-containing bone grafts. J Bone Joint Surg Br. 1964;46:110-40.
- Bolander ME, Balian G. The use of demineralized bone matrix in the repair of segmental defects. Augmentation with extracted matrix proteins and a comparison with autologous grafts. J Bone Joint Surg Am. 1986;68(8):1264-74.
- Sandor GK, Kainulainen VT, Queiroz JO, Carmichael RP, Oikarinen KS. Preservation of ridge dimensions following grafting with coral granules of 48 post-traumatic and post-extraction dento-alveolar defects. Dent Traumatol. 2003;19(4):221-7.
- Senn N. On the healing of aseptic bone cavities by implantation of antiseptic decalcified bone. Am J Med Sci. 1889;98(3):219-47.
- Huggins CB. The formation of bone under the influence of epithelium of the urinary tract. Arch Surg. 1931;22(3):377-408.
- Urist MR. Bone: formation by autoinduction. Science. 1965;150(698):893-9.
- Huggins C, Wiseman H, Reddi AH. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J Exp Med. 1970;132(6):1250-8.
- Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, et al. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990;87(24):9843-7.
- Mikulski AJ, Urist MR. Collagenase-released non-collagenous proteins of cortical bone matrix. Prep Biochem. 1977;7(5):357-81.
- Urist MR, DeLange RJ, Finerman GA. Bone cell differentiation and growth factors. Science. 1983;220(4598):680-6.
- Hu ZM, Peel SA, Sandor GK, Clokie CM. The osteoinductive activity of bone morphogenetic protein (BMP) purified by repeated extracts of bovine bone. Growth Factors. 2004;22(1):29-33.
- Clokie CM, Urist MR. Bone morphogenetic protein excipients: comparative observations on poloxamer. Plast Reconstr Surg. 2000;105(2):628-37.
- Kirker-Head CA, Nevins M, Palmer R, Nevins ML, Schelling SH. A new animal model for maxillary sinus floor augmentation: evaluation parameters. Int J Oral Maxillofac Implants. 1997;12(3):403-11.
- Watanabe K, Niimi A, Ueda M. Autogenous bone grafts in the rabbit maxillary sinus. Oral Surg Oral Med Oral Path Oral Radiol Endod. 1999;88(1):26-32.
- Schenk RK, Buser D, Hardwick WR, Dahlin C. Healing pattern of bone regeneration in membrane-protected defects: a histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994;9(1):13-29.
- Nevins M, Kirker-Head C, Nevins M, Wozney JA, Palmer R, Graham D. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodontics Restorative Dent. 1996;16(1):8-19.
- Ueda M, Tohnai I, Nakai H. Tissue engineering research in oral implant surgery. Artif Organs. 2001;25(3):164-71.
- Wada K, Niimi A, Watanabe K, Sawai T, Ueda M. Maxillary sinus floor augmentation in rabbits: a comparative histologic-histomorphometric study between rhBMP-2 and autogenous bone. Int J Periodontics Restorative Dent. 2001;21(3):252-63.
- Butterfield KJ, Bennett J, Gronowicz G, Adams D. Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg. 2005;63(3):370-6.
- Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003;8(1):328-43.
- Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res. 2002;13(4):405-9.
- Boyne PJ, Marx RE, Nevins M, Triplett G, Lazaro E, Lilly LC, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int J Periodontics Restorative Dent. 1997;17(1):11-25.
- Jurisic M, Markovic A, Radulovic M, Brkovic BM, Sandor GK. Maxillary sinus floor augmentation: comparing osteotome with lateral window immediate and delayed implant placements. An interim report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(6):820-7. Epub 2008 Jul 7.
