ABSTRACT
Metabolic syndrome is associated with increased risk of cardiovascular disease and type 2 diabetes mellitus. However, clinical diagnosis of metabolic syndrome alone does not permit proper assessment of cardiometabolic risk. Instead, it should be viewed as a modifiable risk factor. The development and progression of metabolic syndrome may be the result of many elements, with visceral obesity as a central component. Both metabolic syndrome and visceral obesity are linked to chronic inflammation, which leads to atherosclerosis and, thus, increases cardiometabolic risk. Obesity has been related to caries, traumatic injury, hyposalivation, tooth loss and periodontal diseases, and the latter have been associated with almost every feature of atherosclerosis. Thus, severe or refractory periodontal disease or a significant loss of teeth could serve as markers of cardiometabolic risk. Finally, there is growing evidence that saliva can reflect virtually the entire spectrum of normal and disease states; thus, we are likely to see increased use of saliva as a diagnostic tool and consequently, dentists may have a greater involvement in the identification and monitoring of non-oral disorders.
Introduction
Metabolic syndrome is a cluster of factors associated with risk of developing type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD).1 According to the International Diabetes Federation, metabolic syndrome is defined by the presence of visceral obesity (based on ethnicity-specific waist circumference criteria) plus any 2 of the following factors: hypertriglyceridemia, reduced high-density lipoprotein (HDL) cholesterol levels, hypertension and raised fasting glycemia (or T2DM).2
However, a clinical diagnosis of metabolic syndrome alone does not provide an assessment of cardiometabolic risk.3 The latter (Fig. 1) may be defined as total risk of CVD resulting from the presence of traditional risk factors (age, family history, gender, smoking, high blood pressure, high cholesterol and diabetes) combined with the possible contribution of metabolic syndrome and non-traditional risk factors (genetic factors, prothrombotic state and inflammatory profile).4 According to this definition, metabolic syndrome is a modifiable risk factor rather than a means to assess overall cardiometabolic risk.
Click thumbnail to see full-size image 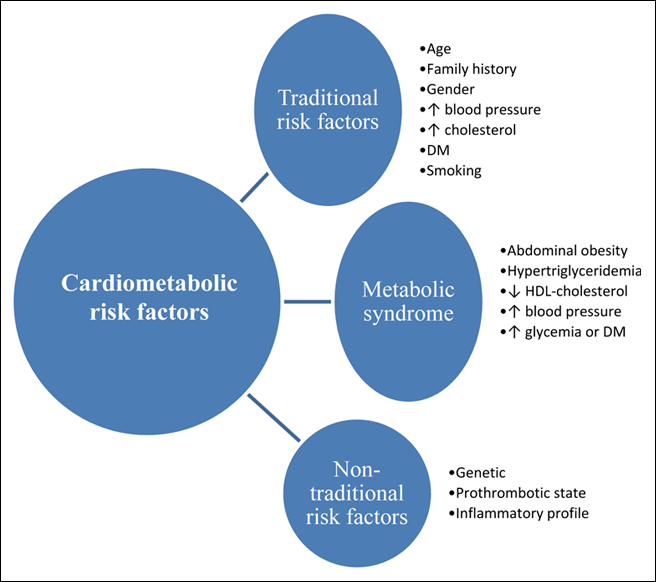 Figure 1: Assessment of cardiometabolic risk. Note: DM = diabetes mellitus, HDL = high-density lipoprotein
Figure 1: Assessment of cardiometabolic risk. Note: DM = diabetes mellitus, HDL = high-density lipoprotein
Metabolic syndrome has received worldwide attention in the past few years because of its increasing prevalence: 20% to 30% of the adult population in almost all countries.5 Metabolic syndrome is insidious in its development and is often diagnosed at a late stage, when signs and symptoms compel the affected person to seek medical care. For the most part, affected people ignore their condition, making preventive intervention of limited use. Thus, there is a need to find new ways to allow early assessment of cardiometabolic risk, and this cannot be achieved without the involvement of all first-line health care professionals.4
It has long been established that CVD and its treatment affect oral health.6 In addition, numerous epidemiologic studies have associated common oral diseases, such as caries, xerostomia (hyposalivation), periodontal diseases and edentation (loss of teeth), with most elements of metabolic syndrome, T2DM and CVD.7 Although these associations are not well understood, various physiologic mechanisms have been proposed to explain them. This suggests that dentists could be involved in the primary prevention and early assessment of cardiometabolic risk. Moreover, the close and regular relationship they maintain with an important part of the population over many years makes them valuable allies.8 The objective of this article is to review the strength of evidence relating to oral diseases, metabolic syndrome and CVD and suggest some potential new oral markers of cardiometabolic risk.
Metabolic Syndrome, Cardiometabolic Risk and Inflammation
Metabolic syndrome results from complex interactions between various genetic and environmental factors. It contributes to atherosclerosis through a process of chronic and incremental damage to the arterial wall associated with hypercoagulability, oxidative stress, endothelial dysfunction and inflammation. Among these elements, the inflammatory process is of particular interest.9,10
Traditionally viewed as a lipid-based disorder, atherosclerosis is now considered to be an inflammatory disease. Not only does inflammation promote the initiation and evolution of atherosclerosis, but it also decisively contributes to precipitation of its acute complications. Indeed, a substantial proportion of cardiovascular events occur in people without the traditional risk factors and may be explained by the systemic low-grade inflammatory state associated with the presence of metabolic syndrome.9,10
Inflammation and Obesity
Obesity is the most prevalent nutritional disorder in industrialized countries.11 However, there is a considerable heterogeneity in the associated health risk based on distribution of adiposity. Whereas accumulation of visceral adipose tissue is an important predictive factor of atherogenic disturbances, adipose tissue in the lower body is less associated with increased cardiometabolic risk.4
This explains the International Diabetes Federation's recommendation to measure waist circumference rather than body mass index (BMI) and recognizes visceral obesity as a central component of the metabolic syndrome. Indeed, visceral adipose tissue, first thought to be an inert storage organ, is now viewed as a producer of a variety of molecules, named adipokines. These molecules have specific biologic functions12-17 and affect energy metabolism by acting in an autocrine, paracrine and endocrine manner. Once in the portal circulation, adipokines are drained to the liver where they modulate the synthesis of hepatic proteins and trigger the development of an inflammatory milieu.18 They have been linked to insulin resistance and vascular disorders.19,20 These problems may be further exacerbated by the presence of traditional cardiometabolic risk factors.21
Commonly associated with such risk factors as dyslipidemia, T2DM and hypertension, obesity is now also known to interact with the inflammatory system. As both obesity and oral health are affected by nutrition, it is plausible that the oral condition reflects the systemic proinflammatory state associated with visceral obesity.
Obesity and Oral Health
There is a close relation between obesity and oral health. Obesity has been related to caries, hyposalivation and periodontal disease, all of which may cause loss of teeth, which may, in turn, alter food selection and contribute further to obesity.22
Epidemiologic studies find that edentulous men and women have, on average, higher BMI and greater visceral obesity than the dentate.22 On one hand, tooth loss has been associated with diminished masticatory performance, food acceptability and perceived ease of chewing, which promote an obesogenic diet associated with higher intake of saturated fats or trans fatty acids rather than a healthy diet containing fibre, vegetables and fruits.7 On the other hand, dental caries and tooth loss may also be the result of inadequate nutrition or lack of health awareness, which are often associated with fat accumulation.7
Obesity has also been associated with hyposalivation (reduced flow of saliva).23 Although the causal relationship remains unknown, many explanations have been proposed, including an alteration in the major salivary glands similar to changes observed in the pancreas24; an inflammatory response in the salivary glands which affects their output capacity25; and an alteration of the hypothalamic–pituitary–adrenal axis and neuroendocrine regulation of the salivary glands due to the immune response.26 Because saliva is essential for adequate functions such as chewing and swallowing, hyposalivation may trigger many oral problems. Normal salivation initiates digestion, enhances healing of mucosa and maintains oral flora balance. It diminishes the risk of developing dental decay through its buffer capacity. Saliva also protects tissues; without its lubricating effects, dentures do not adequately adhere to tissues and the oral mucosa becomes chafed and irritated.27
Very low saliva flow rate has been associated with a BMI > 25 in a cohort of adults under 50 years of age.28 Furthermore, a reduced flow rate has been associated with childhood obesity29 and T2DM.30 These results indicate that a decrease in salivary flow may be the consequence of obesity or its associated systemic diseases rather than a local condition. Salivary hypofunction may also be the result of medications used to treat metabolic syndrome components, such as aspirin and antihypertensive and diuretic drugs.31
These observations have triggered an interest in sialometry (measurement of salivary secretion), and saliva is now increasingly being used in the monitoring of systemic diseases, particularly hypertension and T2DM.24,32 Finally, it has been suggested that obesity, by its effect on immune parameters, could increase susceptibility to periodontal diseases.33
Association Between Periodontal Diseases and Cardiometabolic Risk
Periodontal diseases are a group of common chronic infections that produce an exaggerated inflammatory response to the oral pathogenic microflora. They affect the attachment of connective tissue and the supporting bone around the teeth, leading to tooth mobility and subsequent tooth loss.34 In the past decade, the links between periodontal diseases and CVD have received growing attention because they share common risk factors, such as age, smoking, stress, low socioeconomic status, visceral obesity, diabetes and lack of health awareness.35-37 Nevertheless, some observations suggest the involvement of pathophysiologic mechanisms beyond these traditional risk factors.
For a long time, periodontal diseases have been viewed as local infections with rare systemic implications. However, recently, the remote effects of periodontal diseases on systemic diseases have been investigated, and it has been suggested that this relation may be multidirectional (Fig. 2).38 There is evidence that the inflamed epithelium of periodontal lesions forms an easy entry point that contributes to the dissemination of bacteria, their endotoxins (lipopolysaccharides) and all kinds of immune mediators that are produced locally.35 Once in the circulation, they can exert effects on distant organ systems. In addition, studies have found positive associations between periodontal diseases and intima media thickness and dyslipidemia.36,39 Moreover, common periodontal pathogens have been found in arterial plaques after endarterectomy,40 and successful periodontal treatments appear to have beneficial effects on the control of diabetes, an important cardiometabolic risk factor.41 Considering that obesity, metabolic syndrome, CVD and T2DM are major health problems worldwide, their association with periodontal diseases may be of great importance and deserves to be assessed and investigated in dental clinics.
Click thumbnail to see full-size image 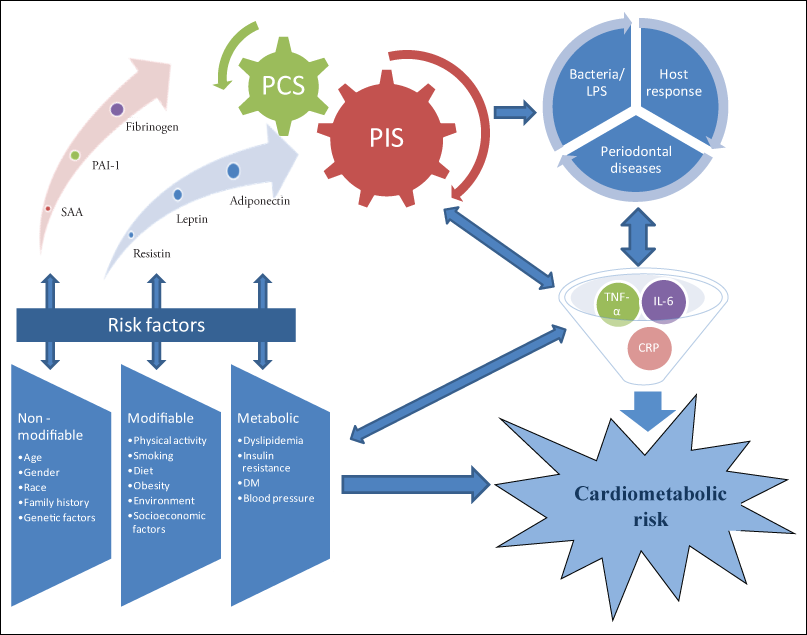 Figure 2: Relation between periodontal diseases and cardiometabolic risk. Note: CRP = C-reactive protein, DM = diabetes mellitus, IL-6 = interleukin-6, LPS = lipopolysaccharides, PAI-1= plasminogen activator inhibitor 1, PCS = procoagulation state, PIS = proinflammatory state, SAA = serum amyloid A, TNF = tumour necrosis factor.
Figure 2: Relation between periodontal diseases and cardiometabolic risk. Note: CRP = C-reactive protein, DM = diabetes mellitus, IL-6 = interleukin-6, LPS = lipopolysaccharides, PAI-1= plasminogen activator inhibitor 1, PCS = procoagulation state, PIS = proinflammatory state, SAA = serum amyloid A, TNF = tumour necrosis factor.
Oral Markers and Cardiometabolic Risk
Despite the bulk of epidemiologic, experimental and clinical evidence supporting the association between oral health and cardiometabolic risk factors, there is little information about oral biomarkers that could be used to assess cardiometabolic risk in clinical dental practice. Edentation should be considered, specifically when it is associated with visceral obesity.7,22 Beyond tooth restoration and prosthetic adjustment, preventive care should include information about nutrition and exercise to improve overall health.42 Because of the propensity of people with metabolic syndrome to develop CVD, dentists must be aware of the close correlation between visceral obesity, a central component of metabolic syndrome, and periodontal diseases.43
People with severe chronic periodontal diseases represent 10%–15% of the population. Moreover, according to one study, approximately 50% of the variation in clinical severity of periodontal diseases would be due to genetic influence.44 However, some researchers have also stated that oral inflammation that is unresponsive to the usual cleaning measures may indicate undiagnosed or uncontrolled T2DM.45 Therefore, severe and treatment-refractory periodontal diseases could serve as oral markers of cardiometabolic risk.
Dentists should also be aware of the risk of systemic complications that may occur during dental procedures, question patients about their history and their symptoms and, if in doubt, consider referring these patients for medical evaluation before any invasive or stressful treatment. However, it is important to note that periodontal treatment leads to clinically relevant improvements in systemic inflammation and should be carried out as early as possible. Because the diagnostic criteria for periodontal diseases are not sufficiently specific, other oral markers should be investigated.
Recently, there has been growing appreciation that saliva can reflect virtually the entire spectrum of normal and disease states.46 Many studies have shown that saliva is a reliable substance for diagnosing levels of steroids, hormones, drugs and antibodies,47,48 and salivary diagnostics is an emerging field that has seen several important developments in the past decade.49 Some of these may be relevant for the prognosis, diagnosis and management of periodontal diseases50,51 and CVD.52 For example, a saliva-based biomarker panel including C-reactive protein, myoglobin and myeloperoxidase has exhibited highly significant diagnostic capability for acute myocardial infarction.49 Although these results remain to be validated for clinical applications, they suggest that saliva could be used to measure the levels of cardiometabolic risk markers in the near future and demonstrate the potential to identify and manage diseases.
Conclusions
Canadian dental clinics form a country-wide network for preventive care and dental treatment.53 They are visited regularly by almost 80% of the general population,54 and dentists routinely treat patients who do not have signs and symptoms of systemic diseases. In contrast, physicians are more likely to see their patients only when they present clinical manifestations of a systemic disease. Thus, Canadian dental clinics could assess public health issues other than oral diseases and successfully carry out cardiometabolic risk-factor screening. Once the diagnostic tools are validated, saliva collection could be carried out by the dental clinic network with the aim of reaching most of the general population.
Moreover, dentists could also be involved in screening and monitoring such common systemic disorders as metabolic syndrome, CVD and T2DM to reduce cardiometabolic risk in the population. We are thus moving from an era of single risk-factor intervention to a holistic management approach with multiple risk-factor interventions. As the fight against the metabolic syndrome epidemic will be difficult, many health professionals must be involved and, in this regard, dentists seem particularly well positioned.
THE AUTHORS
References
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch Intern Med. 2003;163(4):427-36.
- Alberti KG, Zimmet P, Shaw J. Metabolic syndrome — a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469-80.
- Kahn R, Buse J, Ferrannini E, Stern M; American Diabetes Association; European Association for the Study of Diabetes. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28(9):2289-304.
- Després JP, Cartier A, Côté M, Arsenault BJ. The concept of cardiometabolic risk: bridging the fields of diabetology and cardiology. Ann Med. 2008;40(7):514-23.
- Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28(4):629-36. Epub 2008 Jan 3.
- Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med. 2004;15(6):403-13.
- Friedlander AH, Weinreb J, Friedlander I, Yagiela JA. Metabolic syndrome: pathogenesis, medical care and dental implications. J Am Dent Assoc. 2007;138(2):179-87; quiz 248.
- Summary report of the findings of the oral health component of the Canadian Health Measures Survey 2007–2009. Ottawa : Health Canada; 2010. HC Pub. : 100185. (accessed Aug. 29, 2011).
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420(6917):868-74.
- Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med. 1999;340(2):115-26.
- Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xi, 1-253.
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579-91.
- Kusminski CM, McTernan PG, Kumar S. Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond). 2005;109(3):243-56.
- Kusminski CM, Scherer PE. The road from discovery to clinic: adiponectin as a biomarker of metabolic status. Clin Pharmacol Ther. 2009;86(6):592-5.
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4(5):371-9.
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116(1):115-24. Epub 2005 Dec 8.
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972-8.
- Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327-34.
- Samaras K, Botelho NK, Chisholm DJ, Lord RV. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obesity (Silver Spring). 2010;18(5):884-9. Epub 2009 Dec 17.
- Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, et al. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105(9):906-11. Epub 2009 Sep 17.
- Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, et al. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(1):E146-50. Epub 2010 Nov 3.
- Mathus-Vliegen EM, Nikkel D, Brand HS. Oral aspects of obesity. Int Dent J. 2007;57(4):249-56.
- Flink H, Bergdahl M, Tegelberg A, Rosenblad A, Lagerlöf F. Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Community Dent Oral Epidemiol. 2008;36(6):523-31. Epub 2008 Apr 14.
- Chávez EM, Borrell LN, Taylor GW, Ship JA. A longitudinal analysis of salivary flow in control subjects and older adults with type 2 diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(2):166-73.
- Streckfus C, Bigler L, O'Bryan T. Aging and salivary cytokine concentrations as predictors of whole saliva flow rates among women: a preliminary study. Gerontology. 2002;48(5):282-8.
- Pasquali R, Vicennati V, Cacciari M, Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci. 2006;1083:111-28.
- Yurdukoru B, Terzioğlu H, Yilmaz T. Assessment of whole saliva flow rate in denture wearing patients. J Oral Rehabil. 2001;28(1):109-12.
- Powers PS, Holland P, Miller C, Powers HP. Salivation patterns of obese and normal subjects. Int J Obes. 1982;6(3):267-70.
- Modéer T, Blomberg CC, Wondimu B, Julihn A, Marcus C. Association between obesity, flow rate of whole saliva, and dental caries in adolescents. Obesity (Silver Spring). 2010;18(12):2367-73. Epub 2010 Mar 25.
- Ritter AV. Diabetes and oral health. J Esthet Restor Dent. 2007;19(1):63-4.
- Sreebny LM. Saliva in health and disease: an appraisal and update. Int Dent J. 2000;50(3):140-61.
- Streckfus CF. Salivary function and hypertension: a review of the literature and a case report. J Am Dent Assoc. 1995;126(7):1012-7.
- Van Dyke TE. Inflammation and periodontal diseases: a reappraisal. J Periodontol. 2008;79(8 Suppl):1501-2.
- Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36(6):458-67.
- Beck JD, Offenbacher S. Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol. 2005;76(11 Suppl):2089-100.
- Friedewald VE, Kornman KS, Beck JD, Genco R, Goldfine A, Libby P, et al. The American Journal of Cardiology and Journal of Periodontology Editors' Consensus: periodontitis and atherosclerotic cardiovascular disease. Am J Cardiol. 2009;104(1):59-68.
- Saito T, Shimazaki Y, Koga T, Tsuzuki M, Ohshima A. Relationship between upper body obesity and periodontitis. J Dent Res. 2001;80(7):1631-6.
- Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontol 2000. 2007;43:254-66.
- Katz J, Flugelman MY, Goldberg A, Heft M. Association between periodontal pockets and elevated cholesterol and low density lipoprotein cholesterol levels. J Periodontol. 2002;73(5):494-500.
- Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr, Progulske-Fox A. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 2005;25(3):e17-8. Epub 2005 Jan 20.
- Kiran M, Arpak N, Unsal E, Erdoğan MF. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J Clin Periodontol. 2005;32(3):266-72.
- Palacios C, Joshipura K, Willett W. Nutrition and health: guidelines for dental practitioners. Oral Dis. 2009;15(6):369-81. Epub 2009 May 15.
- Saito T, Shimazaki Y, Sakamoto M. Obesity and periodontitis. N Engl J Med. 1998;339(7):482-3.
- Kinane DF, Hart TC. Genes and gene polymorphisms associated with periodontal disease. Crit Rev Oral Biol Med. 2003;14(6):430-49.
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. J Periodontol. 2005;76(11 Suppl):2075-84.
- Segal A, Wong DT. Salivary diagnostics: enhancing disease detection and making medicine better. Eur J Dent Educ. 2008;12 Suppl 1:22-9.
- Hofman LF. Human saliva as a diagnostic specimen. J Nutr. 2001;131(5):1621-5S.
- Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8(2):69-76.
- Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N, et al. Current developments in salivary diagnostics. Biomark Med. 2010;4(1):171-89.
- Miller CS, King CP, Jr, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137(3):322-9.
- Teles RP, Likhari V, Socransky SS, Haffajee AD. Salivary cytokine levels in subjects with chronic periodontitis and in periodontally healthy individuals: a cross-sectional study. J Periodontal Res. 2009;44(3):411-7. Epub 2009 Feb 6.
- Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55:1530-8. Epub 2009 Jun 25.
- National population health survey: health institutions component, longitudinal (NPHS). Ottawa: Statistics Canada; 1999. (accessed Aug. 29, 2011).
- Le Petit C, Berthelot JM. Obesity — a growing issue. Health Rep. 2006;17(3)43-50.
