ABSTRACT
Objective: To describe and evaluate the effectiveness of oral and dental prevention strategies for cancer patients who were about to begin bisphosphonate (BP) intravenous therapy with zoledronate.
Methods: Patients were divided into 2 groups according to their history with BPs: group PA (preventive approach) and group OB (observation). Group PA patients had never been previously treated with BPs, and group OB patients had already undergone therapy with BPs. All patients received a complete oral and dental examination and had a panoramic radiograph. If necessary, oral hygiene, and restorative and rehabilitation therapy were offered to patients. All patients participated in regular checkups every 6 months. Group PA patients underwent oral surgical procedures, as needed.
Results: A total of 282 patients (162 women, 120 men) were included in this analysis (PA: n = 217; OB: n = 65). In group OB, 4.6% of patients presented with osteonecrosis of the jaw (ONJ) at first visit and 10.8% developed new ONJ during the 18-month follow-up period. No patients in group PA had ONJ. Anti-angiogenic therapy was associated with ONJ (p < 0.01) and patients with a higher number of zoledronate infusions were significantly more likely to develop ONJ (p < 0.01).
Conclusion: Although the incidence of BP-related ONJ is fairly low, it remains a painful and difficult complication to treat. An interdisciplinary preventive approach is essential to prevent and manage this condition.
Introduction
Osteonecrosis of the jaw (ONJ) is a rare pathologic condition, which has many possible causes.1 Reported risk factors are having head and neck radiotherapy, chemotherapy, periodontal disease, edentulous regions, dental procedures involving bone surgery and trauma from poorly fitting dentures.2-4 Since 2003, numerous reports have associated ONJ with intravenous (IV) and oral bisphosphonate (BP) therapy.5 BPs are pyrophosphate analogs that generally reduce bone resorption and turnover.6 These drugs have been used for resorptive bone diseases, including Paget's disease of bone, hyperparathyroidism, osteogenesis imperfecta, osteoporosis, and hypercalcemia associated with malignancies; and for a variety of patients with metastatic bone lesions from breast cancer, prostate cancer and multiple myeloma.7,8
The particular BP drug used in therapy depends on the required potency and medical condition. For patients with osteoporosis, oral BPs are used, whereas for cancer patients with bone or skeletal metastases more potent IV BPs are used.9,10 BPs differ one from another in the substitution of the active side chains on their phosphorous-carbon-phosphorous structural backbone. The first generation of BPs (clodronate, etidronate) possesses alkyl or halide side groups. The second generation (pamidronate) is characterized by an amino-terminal moiety and has 10 to 100 times greater antiresorptive potency than first generation BPs. Zoledronate is a representative of third generation BPs that possess an imidazole ring in the side chain. Zoledronate is about 100 times more potent than pamidronate and more than 4 orders of magnitude stronger than first generation BPs.11
According to the position paper of the American Association of Oral and Maxillofacial Surgeons,12 patients are considered to have BP-related osteonecrosis of the jaws (BRONJ) if all 3 of the following characteristics are present: 1) current or previous treatment with a BP, 2) exposed, necrotic bone in the maxillofacial region that has persisted for more than 8 weeks and 3) no history of radiation therapy to the jaws (Fig. 1). Diagnosis may be delayed because, initially, BRONJ has no specific radiographic features.9 The main clinical signs and symptoms of BRONJ are pain, mucosal swelling, areas of exposed and necrotic bone, tooth mobility, erythema, and ulceration, abscesses and fistulas.13 Because of a lack of data about BRONJ,14 the aim of the present study was to evaluate the effectiveness of preventive strategies in 217 patients who underwent therapy with BPs at the department of dentistry in Riuniti di Bergamo Hospital.
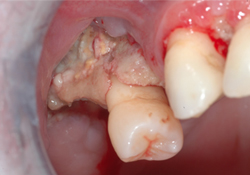 Figure 1: Bisphosphonate-related exposed necrotic bone in the right anterior maxilla in a 70-year-old woman.
Figure 1: Bisphosphonate-related exposed necrotic bone in the right anterior maxilla in a 70-year-old woman.
Methods
Based on the recommendations of the American Association of Oral and Maxillofacial Surgeons' task force on BRONJ,12 282 patients were referred from the department of oncology to the department of dentistry, from January 2007 to November 2008, to undergo dental evaluation and receive necessary treatment before initiating IV therapy with zoledronate. All patients in the study provided written informed consent before the interview and visit. Information about their demographic characteristics, smoking habits, lifetime alcohol consumption and health history was collected.
Statistical Analysis
The distribution of patients' characteristics, including demographics, medical history and therapy, was evaluated. The presence of ONJ was stratified by smoking status, medical history and number of zoledronate infusions; significant heterogeneity was assessed with the chi-square test. All p values reported were considered to be statistically significant at p < 0.05.
Results
A total of 282 patients (162 women, 120 men) were included in this analysis (Table 1). Overall, subjects ranged in age from 30 to 75 years; the median age was 67 years. The majority (275/282, 95.7%) of patients in the study were Italian. Less than 50% (137/282) of patients were affected by breast cancer, followed by prostate (18.8%, 53/282), lung (14.2%, 40/282) and kidney (6.0%, 17/282) cancer. None of the patients reported coagulopathies (data not shown).
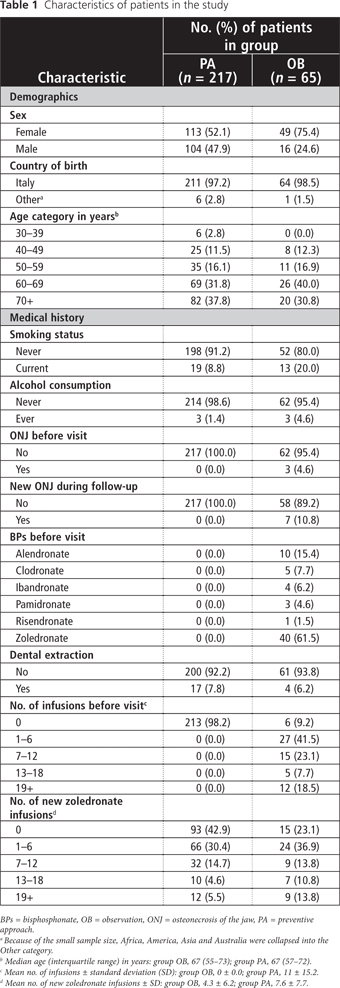
Sixty-five (23.0%) of the 282 patients underwent previous therapy with BPs (group OB) (Table 1). Of these 65 patients, 13 (20.0%) reported current smoking and 3 (4.6%) reported alcohol consumption; 3 (4.6%) had diabetes. Forty (61.5%) patients received IV zoledronate for a median duration of therapy of 8 months, a mean of 11 infusions and a mean cumulative dose of 4 mg. Three (4.6%) had BRONJ at their first visit, and 7 (10.8%) developed new BRONJ during the 18-month follow-up period. Dental extractions were done for 4 (6.2%) of these 65 patients in group OB.
Nineteen (8.8%) of the 217 patients in group PA reported current smoking, 3 (1.4%) reported alcohol consumption and 10 (4.6%) had diabetes (Table 1). None of the patients in group PA developed BRONJ in the following 18 months. Dental extractions were done for 17 (7.8%) of these patients.
The mean number of new zoledronate infusions for patients in group PA and group OB was 4.3 and 7.6, respectively (Table 1).
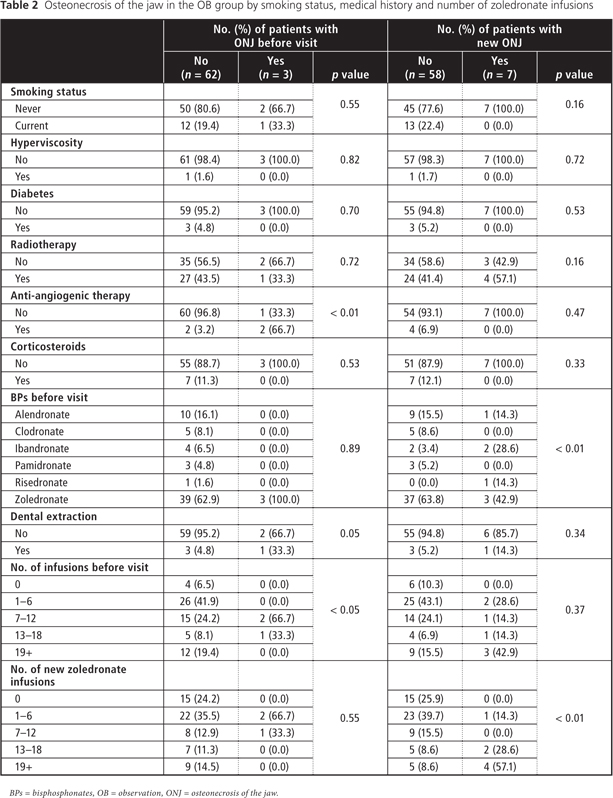
Stratified analyses (Table 2) of those patients in group OB who had ONJ at first oral examination (3 patients) showed that anti-angiogenic therapy (sunitinib malate) was significantly associated with ONJ (66.7% versus 33.3%; p < 0.01). Also, patients with higher infusions of BPs were more likely to develop ONJ (p < 0.05). In the group with new ONJ, BRONJ was observed in 1 patient who previously underwent BP therapy with alendronate; in 2 patients, with ibandronate; in 1 patient, with risedronate; and in 3 patients, with zoledronate (p < 0.01). Patients with a higher number of zoledronate infusions were significantly more likely to develop BRONJ (p < 0.01). No significant associations with ONJ were observed for the following variables: sex, age, smoking status, alcohol consumption, hyperviscosity, diabetes, radiotherapy, corticosteroid therapy and dental surgical procedures.
Discussion
In this study, all patients received BPs because they had oncological diseases. Although the mechanism of BRONJ is still not clear, many reports ascribe a causative role to therapy with BPs.16 The cumulative incidence of BRONJ in patients receiving IV BPs for malignant disease ranges from 0.8% to 12.0%.12 In the current study, 7 (10.8%) of 65 patients in group OB developed BRONJ. None of the patients in group PA developed BRONJ, in contrast to the findings of previous studies.17,18 The results of the current study had a lower incidence rate of BRONJ than those reported by Boonyapakorn and colleagues17 (28.0%, n = 80) and by Badros and colleagues18 (24.5%, n = 90). The preventive strategies adopted for this study may have been effective and reduced the risk of osteonecrosis. Indeed, data from another case-control study19 indicate that preventive dental treatment decreased the risk of BRONJ among patients with malignancy treated with IV BPs.
In the current study, all new cases of BRONJ developed in patients who previously underwent therapy with BPs, and people with a higher number of zoledronate infusions were significantly more likely to develop BRONJ (p < 0.01). Length of exposure seems to be an important risk factor for this complication. Similarly, in a study from Germany,17 the median time of exposure for patients with ONJ was higher than that for patients without ONJ (32 months versus 27 months). Moreover, studies suggest that the incidence of ONJ is related to the type of BP used.20 In the current study, all patients with BRONJ received zoledronate therapy. As suggested by the American Society of Clinical Oncology, zoledronate is one of the most powerful inhibitors of bone resorption within the drug class of BPs and its potency is the basis for the high incidence of BRONJ.21
Type 2 diabetes mellitus is frequently comorbid with ONJ.22 In the current study, 13 (5%) of 282 patients had type 2 diabetes mellitus, none of whom developed ONJ during the 18-month follow-up period. These data differ from those of an Israeli study23 that reported that 58% of their 31 patients with ONJ had type 2 diabetes mellitus. Two cases of ONJ occurred in patients with a history of both BP and anti-angiogenic therapy in our study. Although the numbers are very small, our findings suggest that anti-angiogenic therapy may be another important risk factor for developing ONJ.
One important limitation of this study was the lack of precise data about patients' smoking habits. Heavy smokers are more likely to develop ONJ and are less cooperative during treatment.24 As a consequence, future studies should include detailed data about tobacco smoking to avoid potential biases. Further, given the long-term biologic activity of IV BPs, more follow-up is needed to establish whether new cases of BRONJ may develop.
Although the incidence of BRONJ is fairly low, it remains a painful and difficult complication to treat. An interdisciplinary approach involving dentists, medical oncologists, oral and maxillofacial surgeons, and others is a good strategy to prevent and manage this condition. Also, a preventive regimen involving the maintenance of good oral hygiene should be emphasized. Clinicians' encouragement should help ensure their patients' compliance.
THE AUTHORS
References
- Ruggiero S, Gralow J, Marx RE, Hoff AO, Schubert MM, Huryn JM, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer.J Oncol Pract. 2006;2(1):7-14.
- Assael LA. New foundations in understanding osteonecrosis of the jaws. J Oral Maxillofac Surg. 2004;62(2):125-6.
- Schwartz HC. Osteonecrosis of the jaws: a complication of cancer chemotherapy. Head Neck Surg. 1982;4(3):251-3.
- Tarassoff P, Csermak K. Avascular necrosis of the jaws: risk factors in metastatic cancer patients. J Oral Maxillofac Surg. 2003;61(10):1238-9.
- Ruggiero SL. Bisphosphonate-related osteonecrosis of the jaw: an overview. Ann N Y Acad Sci. 2011;1218:38-46.
- Markiewicz MR, Margarone JE, 3rd, Campbell JH, Aguirre A. Bisphosphonate-associated osteonecrosis of the jaws: a review of current knowledge. J Am Dent Assoc. 2005;136(12):1669-74.
- Watts NB. Treatment of osteoporosis with bisphosphonates. Endocrinol Metab Clin North Am. 1998;27(2):419-39.
- Berenson JR, Rosen LS, Howell A, Porter L, Coleman RE, Morley W, et al. Zoledronic acid reduces skeletal-related events in patients with osteolytic metastases. Cancer. 2001;91(7):1191-200.
- Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosphonate-associated osteonecrosis: an American Academy of Oral Medicine position paper. J Am Dent Assoc. 2005;136(12):1658-68.
- Fournier P, Boissier S, Filleur S, Guglielmi J, Carbon F, Colombel M, et al. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62(22):6538-44.
- Gutta R, Louis PJ. Bisphosphonates and osteonecrosis of the jaws: science and rationale. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(2):186-93. Epub 2007 Apr 20.
- Ruggiero SL, Dodson TB, Assael LA, Landesberg R, Marx RE, Mehrotra B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw — 2009 update. Aust Endod J. 2009;35(3):119-30.
- Ruggiero SL, Drew SJ. Osteonecrosis of the jaws and bisphosphonate therapy. J Dent Res. 2007;86(11):1013-21.
- Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67(5 Suppl):61-70.
- Maerevoet M, Martin C, Duck L. Osteonecrosis of the jaw and bisphosphonates. N Engl J Med. 2005;353(1):99-102.
- Filleul O, Crompot E, Saussez S. Bisphosphonate-induced osteonecrosis of the jaw: a review of 2,400 patient cases. J Cancer Res Clin Oncol. 2010;136(8):1117-24. Epub 2010 May 28.
- Boonyapakorn T, Schirmer I, Reichart PA, Sturm I, Massenkeil G. Bisphosphonate-induced osteonecrosis of the jaws: prospective study of 80 patients with multiple myeloma and other malignancies. Oral Oncol. 2008;44(9):857-69. Epub 2008 Feb 20.
- Badros A, Weikel D, Salama A, Golouveba O, Schneider A, Rapoport A, et al. Osteonecrosis of the jaw in multiple myeloma patients: clinical features and risk factors. J Clin Oncol. 2006;24(6):945-52.
- Dimopoulos MA, Kastritis E, Bamia C, Melakopoulos I, Gika D, Roussou M, et al. Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid. Ann Oncol. 2009;20(1):117-20. Epub 2008 Aug 9.
- Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Falsenberg D, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479-91.
- Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042-57. Epub 2003 Sep 8.
- Favus MJ. Diabetes and the risk of osteonecrosis of the jaw. J Clin Endocrinol Metab. 2007;92(3):817-8.
- Khamaisi M, Regev E, Yarom N, Avni B, Leitersdorf E, Raz I, et al. Possible association between diabetes and bisphosphonate-related jaw osteonecrosis. J Clin Endocrinol Metab. 2007;92(3):1172-5. Epub 2006 Dec 19.
- Sambrook PN, Ebeling P. Osteonecrosis of the jaw. Curr Rheumatol Rep. 2008;10(2):97-101.
