A common oral problem affecting the pediatric population is early childhood caries (ECC), which is defined as the presence of 1 or more decayed, missing, or filled primary teeth in children < 6 years of age.1 ECC poses treatment challenges for very young children, who are unable to cooperate in the delivery of conventional dental treatment in community-based clinical settings.2 Consequently, many children with ECC must be treated under general anesthesia. Manitoba has some of the highest rates of day surgery to treat ECC in Canada, especially in certain northern and inner-city communities.3,4 Unfortunately, although the surgical approach to managing ECC is often needed, it does not address underlying risk factors for caries. In fact, studies have found relapse rates ranging from 22% to 58.5% after treatment for ECC under general anesthesia.5,6 As a result, many children treated for ECC in this manner require repeat general anesthesia, an undesirable outcome given the risks and costs associated with this treatment modality.7
Silver diamine fluoride (SDF) represents a minimally invasive approach to the management of ECC as an alternative to surgical intervention.8 Although SDF has been successfully used in other countries for decades, it was not approved for use in Canada until 2017.9,10 To the best of our knowledge, there are no published studies investigating the use of SDF in the Canadian population, particularly in toddlers and preschool children. Although several systematic reviews support the efficacy of SDF, they all indicate that further research and clinical trials are needed to establish protocols for optimal case selection and its use.11-14
To our knowledge, only 1 other study has investigated the relation between oral-health-related quality of life (OHRQoL) and SDF treatment of ECC.15 This is an important concept to explore, as it may provide valuable insight into how children and parents perceive the effects of this treatment and how it might affect children’s OHRQoL. In addition, such insight may aid in clinical decision-making regarding which patients may benefit from SDF treatment and which patients may be more appropriately managed with conventional surgical treatment.
The aim of our study was twofold: to investigate the feasibility and efficacy of SDF in treating cavitated caries in young children and to explore the association between SDF treatment and OHRQoL.
Methods
This study was a prospective pilot feasibility trial based on a convenience sample. Ethics approval was obtained from the University of Manitoba’s Biomedical Research Ethics Board. Children were recruited from 3 community clinics in Winnipeg, Manitoba, and the study was conducted between June 2017 and November 2018.
Cooperative children < 72 months of age with at least 1 carious primary tooth meeting International Caries Detection and Assessment System (ICDAS) 5 or 6 criteria (i.e., dentinal caries) and without symptoms of pulpal involvement were included. Children allergic to silver or with hereditary dental alterations of teeth were excluded, as were children with severe medical problems or emergent dental needs.
After providing written informed consent, the child’s caregiver completed a baseline questionnaire, via interview, pertaining to child and family demographics, oral hygiene routines, fluoride intake, dietary habits, dental history and appearance of the teeth. Participants underwent a dental examination without radiographs. Eligible cavitated lesions that were accessible were treated with 38% SDF (Advantage Arrest, Oral Science, Brossard, QC, Canada). No caries excavation was performed; however, gross debris was removed. Using cotton isolation, lesions were dried with air or gauze, and a microbrush was used to apply SDF for up to 1 minute, depending on the child’s cooperation. Treated lesions were then rinsed with water or wiped with wet gauze, followed by FV application (NUPRO 5% NaF white varnish, Dentsply Sirona Canada, Woodbridge, ON, Canada). Parents were instructed to have their child avoid eating and drinking for 30 minutes after application and to refrain from brushing teeth until the next morning (because of the fluoride varnish application, which was used for overall caries prevention).
Four months after the baseline visit, participants returned for a second visit during which treated lesions were assessed to determine caries arrest. Lesions that were hard to tactile probing and black in colour were determined to be successfully arrested. A second application of 38% SDF with 5% FV was performed on all previously treated lesions. Parents were asked to complete an Early Childhood Oral Health Impact Scale (ECOHIS) questionnaire. Approximately 4 months after this second visit, participants returned for a third and final visit for assessment of caries arrest and to complete a follow-up questionnaire and a second ECOHIS questionnaire.
The colour (yellow, brown, black) and hardness (very soft, medium, very hard) of treated lesions as well as the dmft score were recorded at baseline and at each follow-up visit. Hardness was assessed by applying light force to the caries lesion with a probe. A single experienced examiner (RJS) was involved throughout the study and was responsible for the application of SDF as well as the assessment of caries arrest. Child level analysis focused on classifying SDF treatment as completely successful if all treated lesions were found to be arrested and incompletely successful if at least 1 lesion was found not to have been arrested. Treated teeth were assessed for the presence of pain and/or infection at each follow-up visit. The behaviour of the child and difficulty encountered in providing treatment was also documented.
The ECOHIS, which was administered by interview, is a validated questionnaire which uses caregiver responses to assess OHRQoL in preschool children and their families.16 ECOHIS consists of 13 questions divided into 2 sections: the child impact section (CIS) which has 4 domains (symptoms, function, psychological, self-image/social interaction) and the family impact section (FIS) which has 2 domains (parent distress, family function)sup Responses were coded according to ECOHIS protocol: 0 = never, 1 = hardly ever, 2 = occasionally, 3 = often, 4 = very often, 5 = don’t know.16 Total scores were calculated as a simple sum of the responses.16 The CIS score can range from 0 to 36 and the FIS from 0 to 16, with the total possible score ranging from 0 to 52.16 A higher score indicates a greater impact and a poorer OHRQoL.
Data from the clinical assessments as well as from the ECOHIS questionnaires were entered into an Excel (Microsoft, Redmond, Wash., USA) database. Statistical analyses were performed using the Number Cruncher Statistical Software Version 12 (Kaysville, Utah, USA). Both descriptive (frequencies and means) and bivariate (t tests, and Fisher’s exact tests) analyses were performed. A p value ≤ 0.05 was significant.
Results
Participant Characteristics
A total of 40 children (45% male) with a mean age of 40.2 ± 14.9 months were recruited (Table 1). Approximately a third identified as refugees or newcomers (defined as < 2 years in Canada). The mean number of ICDAS 5 and 6 lesions treated per child was 6.0 ± 3.8.
|
Characteristic |
No. (%), except where indicated |
|---|---|
|
Note: dmft = decayed, missing, filled teeth, ICDAS = International Caries Detection and Assessment System, SD = standard deviation. |
|
| Sex
Male Female |
|
| Mean age, months ± SD, range | 40.2 ± 14.9, 17–71 |
| Dental Insurance
Yes No Unsure |
|
| Refugee/newcomer
Yes No |
|
| Ethnicity
African Asian Caucasian Other |
|
| Use of fluoridated toothpaste
Yes No Unsure |
|
| Frequency of tooth brushing
Once daily Twice daily Every other day Never |
|
| Baseline dmft, mean ± SD | 5.9 ± 3.2 |
| No. lesions treated per child (ICDAS 5 and 6), mean ± SD | 6.0 ± 3.8 |
Lesion-Level Analysis
At the baseline visit, 239 caries lesions (111 anterior, 128 posterior) were treated with SDF. The treated lesions were located on primary incisors (40.2%), canines (6.3%), first molars (30.5%) and second molars (23.0%). All participants returned for the second visit. It was determined that 74.1% lesions had arrested after 1 application of SDF (Fig. 1). Two children presented with an abscessed SDF-treated tooth and 1 child had an abscessed tooth extracted before the second visit. The mean dmft score increased from 5.9 ± 3.2 at baseline to 6.0 ± 3.3. The mean time elapsed between baseline and the second visit was 16.7 ± 2.8 weeks.
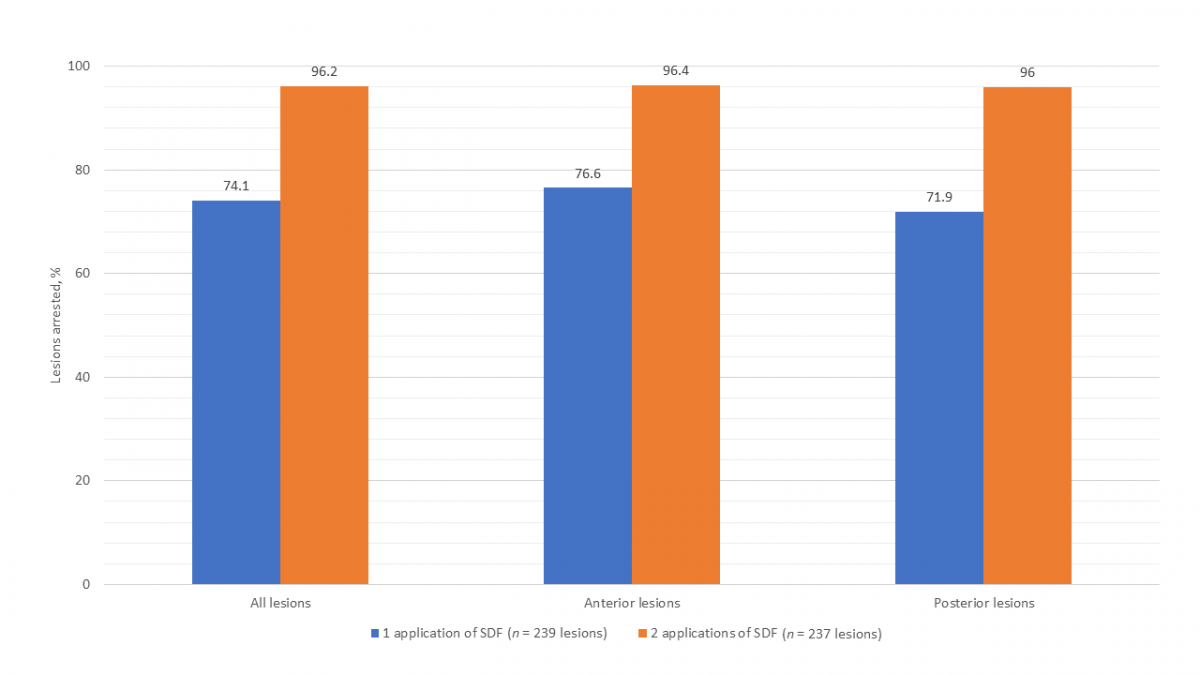
All participants returned for the third and final visit. Two lesions were excluded from analysis as 1 tooth was extracted for an unknown reason before the third visit and another had insufficient data recorded to determine arrest, reducing total lesions to 237 (111 anterior, 126 posterior). It was determined that 96.2% lesions had arrested after 2 applications of SDF (Fig. 1). The mean dmft score increased significantly from 6.0 ± 3.3 at second visit to 6.3 ± 3.3 (paired t test, p = 0.036). The mean time elapsed between the second and third visit was 18.0 ± 3.6 weeks.
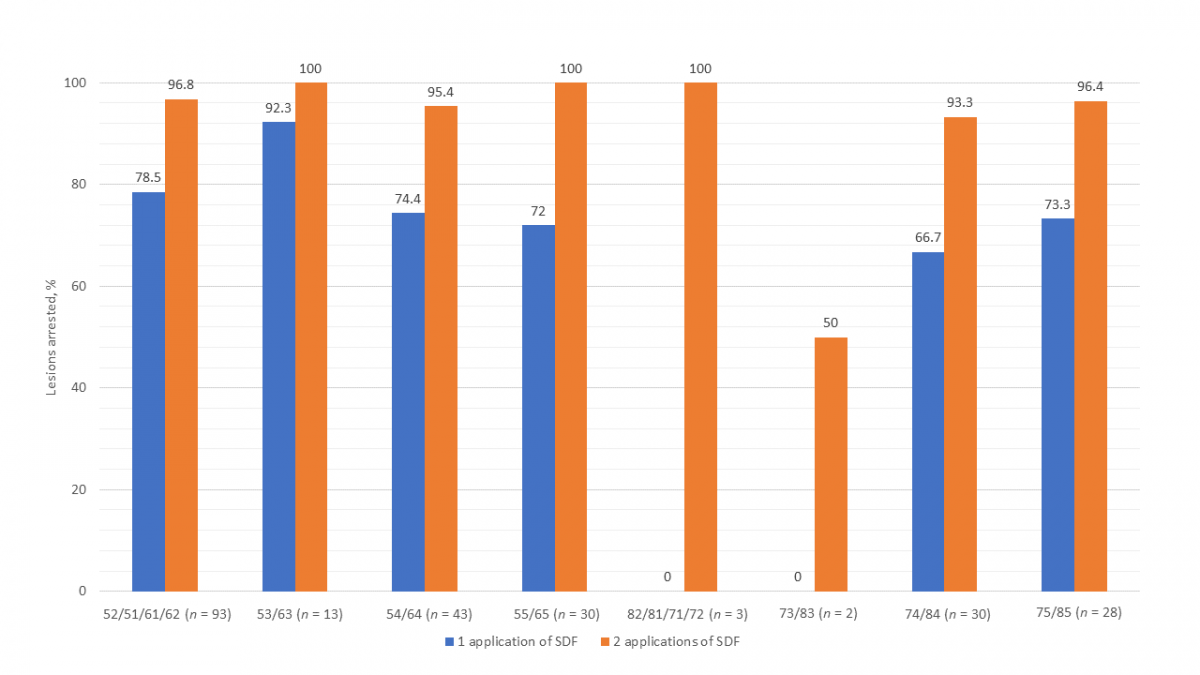
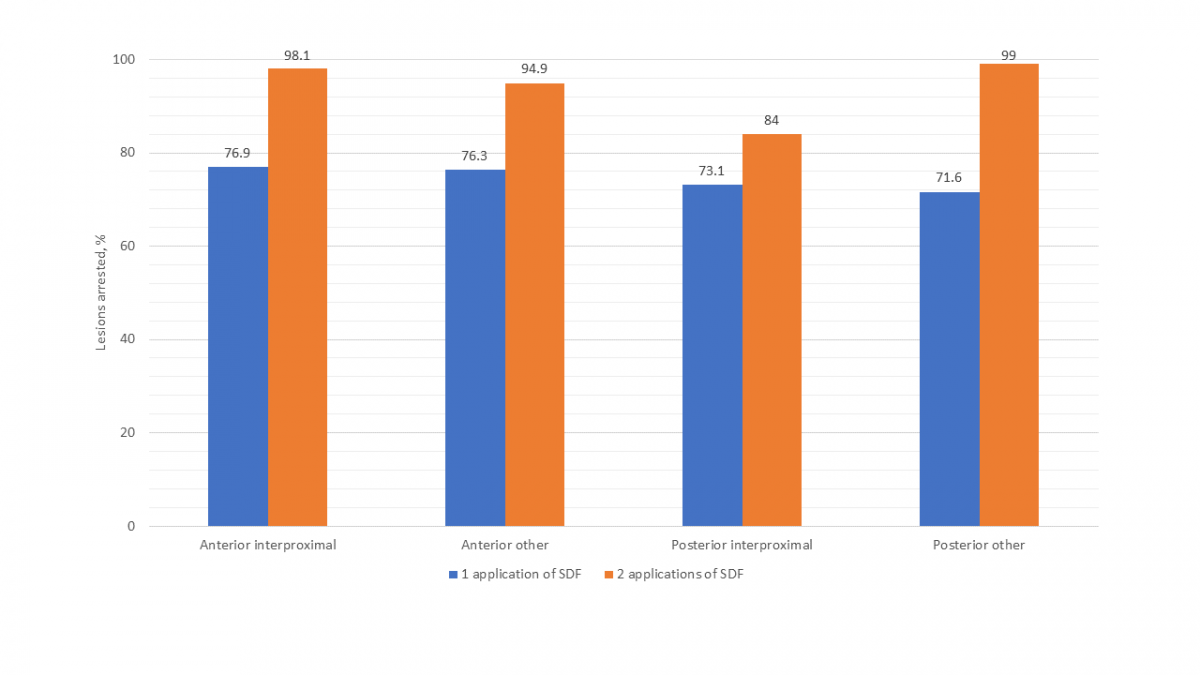
Figure 2 presents the success of SDF by specific primary teeth. Other than the mandibular incisors and canines, which had only 5 lesions, the mandibular first molar had the lowest arrest rate (66.7%) of all teeth after 1 application of SDF. Arrest rates for all teeth were higher after 2 applications of SDF. Although the success of SDF in arresting interproximal lesions versus those on other surfaces was comparable after a single application, after 2 applications, interproximal lesions on posterior teeth were found to have a notably lower arrest rate (Fig. 3).
Child-Level Analysis
At the second visit, 17 (42.5%) participants were determined to be in the completely successful arrest group and 23 (57.5%) in the incompletely successful arrest group. Participants noted above who presented with abscessed teeth following SDF application or for whom arrest data were unknown or missing were classified as incompletely successful. χ2 analyses revealed no significant relation between arrest and sex, frequency of tooth brushing, difficulty providing treatment or use of fluoridated toothpaste (Table 2).
|
Second visit (n = 40) |
Third visit (n = 40) |
|||||
|---|---|---|---|---|---|---|
|
Complete success, no. (%) |
Incomplete success, no. (%) |
p |
Complete success, no. (%) |
Incomplete success, no. (%) |
p |
|
| Sex
Male Female |
|
|
|
|
|
|
| Frequency of tooth brushing
Twice daily < twice daily |
|
|
|
|
|
|
| Difficulty providing treatment
Yes No |
|
|
|
|
|
|
| Use of fluoridated toothpaste
Yes No/unsure |
|
|
|
|
|
|
At the third visit, 34 (85.0%) participants were determined to be in the completely successful arrest group and 6 (15.0%) were found to be in the incompletely successful group. χ2 analysis revealed a significant relation between the frequency of tooth brushing and successful arrest, with brushing twice daily associated with a greater likelihood of being in the completely successful arrest group (p = 0.006). A significant association was observed between baseline dmft score and arrest at third visit, with participants in the completely successful arrest group having a lower mean score of 5.4 compared with 8.8 in the incompletely successful arrest group (2-sample t test, p = 0.048).
ECOHIS
At the second visit, mean total ECOHIS score was 3.8 ± 4.4 out of a possible 52, with mean CIS score 1.9 ± 3.1 out of a possible 36 and mean FIS score 1.9 ± 2.1 out of a possible 16 (Table 3). Third-visit mean scores were 2.7 ± 3.8, 1.3 ± 2.8 and 1.4 ± 2.0, respectively, and were not significantly different from second-visit scores. Only 2 parameters had an increased score: “difficulty drinking” in the CIS and “felt guilty” in the FIS. There was no significant difference in mean total, CIS or FIS scores between completely and incompletely successful groups.
|
Child impact section |
Mean (SD) |
Never, no. (%) |
Hardly ever, no. (%) |
Occasionally, no. (%) |
Often, no. (%) |
Very often, no. (%) |
|---|---|---|---|---|---|---|
| Child symptoms |
0.60 (0.87) |
|||||
| 1. Oral/dental pain |
25 (62.5) |
7 (17.5) |
7 (17.5) |
1 (2.5) |
0 (0) |
|
| Child functions |
0.78 (1.73) |
|||||
| 2. Difficulty drinking |
37 (92.5) |
1 (2.5) |
1 (2.5) |
1 (2.5) |
0 (0) |
|
| 3. Difficulty eating |
35 (87.5) |
2 (5.0) |
1 (2.5) |
1 (2.5) |
1 (2.5) |
|
| 4. Difficulty pronouncing words |
35 (87.5) |
2 (5.0) |
0 (0) |
3 (7.5) |
0 (0) |
|
| 5. Missed preschool or school |
37 (92.5) |
3 (7.5) |
0 (0) |
0 (0) |
0 (0) |
|
| Child psychological factors |
0.33 (0.76) |
|||||
| 6. Trouble sleeping |
37 (92.5) |
2 (5.0) |
1 (2.5) |
0 (0) |
0 (0) |
|
| 7. Irritable or frustrated |
35 (87.5) |
2 (5.0) |
2 (5.0) |
1 (2.5) |
0 (0) |
|
| Child self image/social interaction |
0.23 (0.70) |
|||||
| 8. Avoid smiling or laughing |
36 (90.0) |
2 (5.0) |
2 (5.0) |
0 (0) |
0 (0) |
|
| 9. Avoid talking |
38 (95.0) |
1 (2.5) |
1 (2.5) |
0 (0) |
0 (0) |
|
| Total |
1.93 (3.13) |
|||||
| Family impact section | ||||||
| Parental distress |
1.03 (1.69) |
|||||
| 10. Been upset |
29 (72.5) |
3 (7.5) |
6 (15.0) |
1 (2.5) |
1 (2.5) |
|
| 11. Felt guilty |
31 (77.5) |
1 (2.5) |
6 (15.0) |
2 (5.0) |
0 (0) |
|
| Family function |
0.83 (1.30) |
|||||
| 12. Time off work |
31 (77.5) |
2 (5.0) |
5 (12.5) |
2 (5.0) |
0 (0) |
|
| 13. Financial impact |
32 (80.0) |
2 (5.0) |
5 (12.5) |
1 (2.5) |
0 (0) |
|
| Total |
1.85 (2.07) |
|||||
| Total ECOHIS score |
3.78 (4.44) |
|||||
Discussion
This study was a pilot feasibility trial that sought to investigate the efficacy of SDF with 5% FV in treating cavitated caries in young children and the association with OHRQoL. There are few definitive guidelines for the use of SDF in this population as a nonsurgical alternative to caries management. Our study may contribute to the formation of a protocol that can be used by clinicians to select and treat patients using SDF, particularly those for whom conventional surgical management is difficult (because of special needs, behaviour, young age or lack of access to care), not indicated or delayed (because of long wait times for general anesthesia or administrative barriers).
Our study demonstrated that 2 applications of SDF are more effective than a single application. Although variability in arrest rates was observed among tooth types and location, arrest rates in all situations were higher after 2 applications. Greater success with more frequent applications of SDF has been reported in other studies.17,18 In a 24-month study, Zhi and colleagues17 reported an arrest rate of 79% when SDF was applied every 12 months and 91% when applied every 6 months. A 30-month study by Fung et al.18 reported a 66.9% arrest rate with annual application and 75.7% with biannual application. As a result of these findings, we recommend that patients receive more than 1 application of SDF for optimal results.
In our study, higher arrest rates were observed for anterior (i.e., incisors and canines) than posterior lesions. This finding is consistent with several other studies and may be attributed to the increased accessibility of anterior lesions for cleaning.17,18 Although the arrest rates of lesions located on both interproximal and other (i.e., occlusal, buccal, lingual) surfaces were comparable after a single application of SDF, a notably lower arrest rate was found in posterior interproximal lesions compared with those on other surfaces after 2 applications. Other studies have also found less success treating lesions on interproximal surfaces compared with other surfaces.17,18 This also may be a result of the increased difficulty in cleaning these surfaces as well as the increased likelihood of food impaction to occur there.
Children who reportedly brushed twice daily were more likely to be in the completely successful arrest group at the third visit compared with those who brushed less often. Also, children in the completely successful arrest group at the third visit had a lower baseline dmft compared with those with incompletely successful arrest. One study found that poor oral hygiene, as indicated by a higher visible plaque index (VPI), was associated with a lower arrest rate after annual application of SDF; however, the arrest rate increased with biannual application.18 In contrast, another study found no association between baseline dmft or frequency of tooth brushing and caries arrest.17 This discrepancy may be because tooth brushing is a less accurate measure of oral hygiene than VPI, as it depends on technique and parental report. The results of our study suggest that children with poor adherence to recommended oral hygiene practices and those who have a higher baseline dmft may require more frequent recall and application of SDF. VPI may be a more accurate measure of oral hygiene to include in a future study design.
The increase in dmft observed between visits suggests that, despite treatment with SDF, new caries can still develop on other teeth and surfaces. Although 1 study18 had a similar finding, others found a significant reduction in the development of new caries in children treated with SDF compared with those receiving no treatment.19,20 Although the development of new caries may be reduced with SDF treatment, no studies have demonstrated an absolute reduction. In fact, a recent review of systematic reviews reported that there is insufficient evidence that SDF prevents coronal caries in primary dentition.21 Therefore, it is prudent to emphasize the importance of maintaining good oral hygiene practices in conjunction with SDF treatment, as SDF does not address underlying risk factors for ECC.
No difference in OHRQoL was observed between completely and incompletely successful arrest groups, a finding consistent with another study.15 This is likely because children who had emergent treatment needs were excluded from the study and, therefore, participants had less extensive dental needs and presumably higher OHRQoL initially, making treatment effects less pronounced. This may also explain why ECOHIS scores were low overall. The increase in the parental distress parameter “felt guilty” was also observed in the other study and may be explained by the black staining, which serves as a daily visual reminder to parents of their child’s past caries experience.15 Of note, among all of the parents who were approached to have their child(ren) participate in the study, only 1 declined, which suggests high parental acceptance of SDF treatment. A qualitative component of this study, which will be reported subsequently, might elicit further information on parental views and attitudes toward SDF as a caries management agent.
A limitation of this study was the lack of a baseline ECOHIS assessment. Without this information, a potential decrease in OHRQoL between the baseline and second visit would not be identified. Other limitations include the small sample size, short follow-up period of 8 months and lack of a control group. Because of the obvious black staining resulting from SDF treatment, it was not possible to include a blind observer in the study design. No radiographs were obtained because of the young age and resultant guarded cooperation of the participants. As a result, eligible teeth were selected based on clinical examination, which may have failed to accurately assess the proximity of caries to the pulp in some cases and cause improper case selection. This might explain why 2 children experienced an abscess in an SDF-treated tooth. Study strengths are the longitudinal design with no participant drop out and the high proportion of refugees and newcomers in the sample, representing patients who may benefit most from SDF. The application of 5% NaF may have also influenced our results and added to the benefit of SDF. Another strength is the assessment of OHRQoL, which is an important measure and an essential patient-centred outcome of vital interest to those implementing and disseminating findings.
This study can inform both general and pediatric dentists of optimal patient selection and treatment protocol for the management of ECC with SDF. It can also support the validation and refinement of a protocol and research methods for future investigations and clinical use. SDF may be used in a vast array of situations because of its low cost, simple application and minimal requirement for special equipment. Patients who may not otherwise receive dental care because of financial or geographic barriers could benefit greatly from SDF treatment. SDF may be used as the definitive treatment in situations where a tooth is expected to exfoliate shortly, and the loss of tooth structure does not severely compromise function. Consideration should be given to routinely employing SDF as an adjunct to surgical treatment to slow or halt disease progression while children await general anesthesia. If such use becomes the standard of care, the morbidity associated with ECC could be greatly reduced in that fewer extractions and pulp treatments may be required by the time the child is seen for definitive treatment. SDF can also be used to manage ECC until the child’s age and level of cooperation allow for conventional treatment in a clinical setting, thereby reducing dependence on general anesthesia and alleviating scarce hospital and health care system resources. This would allow for the reallocation of funds to more effective primary and secondary methods of prevention.
Conclusions
In the short term — at least up to 8 months — SDF with 5% FV is an effective secondary prevention approach to the management of ECC. More than 1 application is recommended along with regular follow up of patients. The importance of optimal home care, including twice-daily brushing, must be emphasized in patients undergoing treatment with SDF to maximize efficacy and prevent the development of new caries. Children with relatively low baseline dmft scores may experience greater success with SDF treatment compared with those with higher scores. OHRQoL assessment may guide patient selection and help to differentiate children with less disease severity who may be treated with SDF from those with more severe disease, especially in the molars, who may require more conventional surgical treatment. From the clinical practice perspective, it is essential to perform follow-up studies to determine how SDF-treated children compare with those treated by conventional methods over the long term.
THE AUTHORS
Acknowledgements: Dr. Schroth holds a Canadian Institutes of Health Research (CIHR) Embedded Clinician Researcher salary award in “Improving access to oral health care and oral health care delivery for at-risk young children in Manitoba.” Operating funds for this research were provided through these funds as well as through the Dr. Gerald Niznick College of Dentistry, University of Manitoba. The authors thank the clinical staff at Access Downtown, Mount Carmel Clinic, SMILE plus, for their assistance with this study. Additional thanks are extended to Jeanette Edwards, BOT, MHA, Pamela Dahl, DMD, Geert W ’tJong, Lisette Dufour, RDH, and Khalida Hai-Santiago, DMD, for their contributions.
Correspondence to: Dr. Robert J. Schroth, 507–715 McDermot Ave., Winnipeg MB R3E 3P4.
Email: robert.schroth@umanitoba.ca
The authors have no declared financial interests.
This article has been peer reviewed.
References
- Policy on early childhood caries (ECC): classifications, consequences, and preventive strategies. Pediatr Dent. 2016;38(6):52-4.
- Behavior guidance for the pediatric dental patient. Pediatr Dent. 2018;40(6):254-67.
- Schroth RJ, Quiñonez C, Shwart L, Wagar B. Treating early childhood caries under general anesthesia: a national review of Canadian data. J Can Dent Assoc. 2016;82:g20.
- Schroth RJ, Pang JL, Levi JA, Martens PJ, Brownell MD. Trends in pediatric dental surgery for severe early childhood caries in Manitoba, Canada. J Can Dent Assoc. 2014;80:e65.
- Amin M, Nouri R, ElSalhy M, Shah P, Azarpazhooh A. Caries recurrence after treatment under general anaesthesia for early childhood caries: a retrospective cohort study. Eur Arch Paediatr Dent. 2015;16(4):325-31.
- El Batawi HY. Factors affecting clinical outcome following treatment of early childhood caries under general anaesthesia: a two-year follow-up. Eur Arch Paediatr Dent. 2014;15(3):183-9.
- Schroth RJ, Smith WF. A review of repeat general anesthesia for pediatric dental surgery in Alberta, Canada. Pediatr Dent. 2007;29(6):480-7.
- Clarkson BH, Exterkate RAM. Noninvasive dentistry: a dream or reality? Caries Res. 2015;49 suppl 1:11-17.
- Martel S. Silver diamine fluoride: new in North America! Oral Health. 2017;30 July:36-8.
- Yee R, Holmgren C, Mulder J, Lama D, Walker D, van Palenstein Helderman W. Efficacy of silver diamine fluoride for arresting caries treatment. J Dent Res. 2009;88(7):644-7.
- Gao SS, Zhao IS, Hiraishi N, Duangthip D, Mei ML, Lo ECM, et al. Clinical trials of silver diamine fluoride in arresting caries among children: a systematic review. JDR Clin Trans Res. 2016;1(3):201-10.
- Gao SS, Zhang S, Mei ML, Lo ECM, Chu CH. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment — a systematic review. BMC Oral Health. 2016;16:12.
- Contreras V, Toro MJ, Eliás-Boneta AR, Encarnación-Burgos A. Effectiveness of silver diamine fluoride in caries prevention and arrest: a systematic literature review. Gen Dent. 2017;65(3):22-9.
- Duangthip D, Jiang M, Chu CH, Lo ECM. Non-surgical treatment of dentin caries in preschool children — systematic review. BMC Oral Health. 2015;15:44. doi:10.1186/s12903-015-0033-7.
- Duangthip D, Gao SS, Chen KJ, Lo ECM, Chu CH. Oral health-related quality of life of preschool children receiving silver diamine fluoride therapy: a prospective 6-month study. J Dent. 2019;81:27-32.
- Pahel BT, Rozier RG, Slade GD. Parental perceptions of children’s oral health: the Early Childhood Oral Health Impact Scale (ECOHIS). Health Qual Life Outcomes. 2007;5:6.
- Zhi QH, Lo ECM, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40(11):962-7.
- Fung MHT, Duangthip D, Wong MCM, Lo ECM, Chu CH. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J Dent Res. 2018;97(2):171-8.
- Lo EC, Chu CH, Lin HC. A community-based caries control program for pre-school children using topical fluorides: 18-month results. J Dent Res. 2001;80(12):2071-4.
- Llodra JC, Rodriguez A, Ferrer B, Menardia V, Ramos T, Morato M. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2005;84(8):721-4.
- Seifo N, Cassie H, Radford JR, Innes NPT. Silver diamine fluoride for managing carious lesions: an umbrella review. BMC Oral Health. 2019;19(1):145.